1. Introduction
Pharmaceuticals are substances with a high biological activity, which are introduced into the body in a strictly defined dose to achieve a desired (therapeutic or preventive) effect. They are exposed to the environment in many ways, the most important being excretion by humans and animals and inappropriate disposal of drugs. These compounds are excreted from the body in the form of parent compounds or as metabolites formed in the first and second phase of biotransformation [
1,
2,
3]. Many pharmaceutically active compounds were already detected in water in the 1980s. Bush (1997) grouped these therapeutic substances into the following classes: (a) anti-inflammatory agents and analgesics, (b) antibiotics, (c) antiepileptics, (d) antidepressants, (e) lipid-lowering agents, (f) antihistamines, (g) β-blockers, and (h) other substances [
3,
4].
Pharmaceuticals that are most frequently detected, including antibiotics, anti-inflammatory drugs, and analgesics, have become a growing environmental concern worldwide [
5,
6]. They occur mainly in the aquatic environments, such as surface and underground waters, water reservoirs, effluents and influents of sewage treatment plants, and drinking water [
7,
8,
9,
10,
11,
12,
13]. Medicines are found in trace concentrations up to 100 µg L
−1 in wastewater resulting from drug production [
14]. Drugs are found in the environment because these pollutants cannot be completely removed in sewage treatment plants [
15], and thus persist without undergoing degradation [
16]. Incomplete elimination of pharmaceuticals was also observed in drinking water treatment plants [
17,
18]. This paper focuses on two groups of pharmaceuticals—non-steroidal anti-inflammatory drugs (NSAIDs) and antibiotics—as they are the most widely used medicinal products worldwide. NSAIDs (diclofenac, ibuprofen, ketoprofen, and naproxen) were chosen owing to their large-scale use and widespread distribution in surface waters and wastewater, which is confirmed by numerous scientific studies [
19,
20,
21]. In turn, the antibiotics discussed in this article (erythromycin, sulfamethoxazole, tetracycline, and trimethoprim) were selected as they are included in the World Health Organization’s List of Essential Medicines [
22] and are also used widely as antimicrobial substances against bacteria [
20,
23,
24]. Of the NSAIDs of interest, only ibuprofen is on the WHO list.
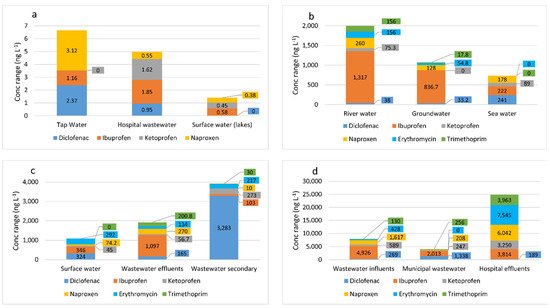
Figure 1a–d presents the concentrations of selected drugs from the group of NSAIDs and antibiotics found in the aquatic environment based on data from the analyzed studies.
Figure 1. Concentrations of some pharmaceuticals (antibiotics, anti-inflammatory drugs, and analgesics) recorded in the world in selected water types: (
a) tap water [
25], hospital wastewater, and surface water (lakes) [
26]; (
b) river water, groundwater [
27], and seawater [
28]; (
c) surface water [
29], wastewater effluents, and wastewater secondary [
27,
30]; and (
d) wastewater influents [
30], municipal wastewater [
29], and hospital effluents [
30].
Based on the data from
Figure 1a–d, it can be stated that antibiotic concentrations were highest in hospital effluents, wastewater effluents, and river water. In three types of water (tap water, hospital wastewater, surface water (lakes)) presented in
Figure 1a, erythromycin and trimethoprim were not detected. The highest concentrations of diclofenac, ibuprofen, and ketoprofen were found in wastewater influents, municipal wastewater, and hospital effluents. Thus, it can be concluded that the drug concentrations in different types of waters and wastewater are found in the following order: hospital effluents > wastewater influents > municipal wastewater > secondary wastewater > river water > wastewater effluents > groundwater > surface water > seawater > tap water > hospital wastewater > surface water (lakes). Owing to human activity, pharmaceuticals are detected in various types of water and wastewater on each continent, including the North Scandinavian water environment [
31]. The strategy for their removal can be the same everywhere, as long as the concentrations are at a similar level. The most easily removable drugs are mainly those belonging to the group of non-steroidal analgesics and anti-inflammatory drugs, including ibuprofen, ketoprofen, and naproxen. On the other hand, the elimination of pharmaceuticals such as diclofenac from wastewater is difficult.
Pharmaceuticals are chemically stable. However, owing to physicochemical and biotic factors [
32], they undergo biodegradation, conjugation, deconstruction, and sorption. Therefore, the knowledge of these processes is necessary to predict the environmental fate of medicinal substances [
13]. The high stability of drugs is related to their relatively high durability under environmental conditions. In contrast, some pharmaceutical metabolites resulting from oxidation, reduction, and/or hydrolysis are more susceptible to further transformations, and thus are less stable in the aquatic environment [
33]. The transformations of pharmaceuticals taking place in the aquatic environment are not thoroughly studied so far [
34,
35,
36]. Pharmaceuticals undergo many reactions and changes, the first of which dilutes the drugs when they reach the surface water and water reservoirs [
37], while chemical reactions may partially or completely change the original pharmaceuticals (parent compounds) [
1]. The products resulting from the transformation of pharmaceutical compounds are sometimes more stable than the parent compounds and may be more or less toxic. Moreover, pharmaceuticals may undergo biotic (aerobic and anaerobic) and abiotic (chemical) reactions in the environment [
15,
38]. Most often, pharmaceuticals are trapped in sewage sludge, but their original molecular structures are preserved. This is generally observed in the case of lipophilic and difficult-to-degrade substances. Pharmaceuticals also possibly transform into hydrophilic compounds, which remain stable. Such hydrophilic products pass through sewage treatment plants and reach the flowing surface waters (rivers) and still surface waters (water reservoirs and lakes) [
5]. It has been shown that pharmaceuticals exhibit a very wide range of removal rates without any logical scheme, even if they belong to the same therapeutic groups [
39].
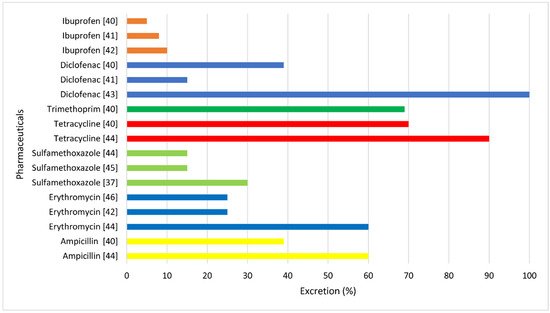
Figure 2 presents the approximate nonmetabolized fractions of selected pharmaceuticals from the NSAID group and that of antibiotics entering wastewater after ingestion and human metabolism. The
x-axis excretion percentage represents unmetabolized or partially metabolized pharmaceuticals that are eliminated as the original active ingredient.
Figure 2. Typical pharmaceuticals and their approximate nonmetabolized fractions entering sewage after being ingested and subjected to human metabolism: ibuprofen [
40,
41,
42]; diclofenac [
40,
41,
43]; trimethoprim [
40]; tetracycline [
40,
44]; sulfamethoxazole [
37,
44,
45]; erythromycin [
42,
46]; and amplicillin [
40,
44].
Pharmaceuticals enter the environment mainly by water transport and further spread into the environment through the food chain [
7]. The side effects of these substances are still unknown and have not been tested. Pharmaceuticals can affect aquatic ecosystems, but the extent of this damage is not clear [
5,
47]. Some studies have already reported that these compounds pose both acute and chronic threats to flora and fauna. It has been proven that diclofenac has a negative effect on vultures, causing a decline in their population [
48]. In turn, Schwaiger et al. [
49] and Triebskorn et al. [
50] indicated that exposure of rainbow trout to diclofenac results in damage to internal organs. Sulfamethaxazole has also been shown to affect the germination of rice and oats [
51].
Because of the above-described consequences, it is necessary to optimize and improve the technologies currently used for the treatment of wastewater and surface water in order to eliminate pharmaceutical residues from them. Because the biological and physical removal efficiency of these residues is not very high, there is a need to search for other more effective cleaning methods. Chemical (e.g., ozonation and oxidation) and physicochemical processes (e.g., adsorption, membrane filtration, and coagulation) are commonly used for the removal of medicines from aqueous solutions [
52,
53,
54]. Some of the pharmaceutical substances in the suspension go to both primary and secondary sediments. Among the proposed physicochemical processes, adsorption is the most preferred method for removing pharmaceutical residues [
55], which works based on the principle of remediation [
3]. The advantages of adsorption are that it allows obtaining high-quality treated wastewater, it is easy and cheap to operate, and it does not result in the production of undesirable by-products [
56,
57]. It can be used for the treatment of various types of water and wastewater, including those with a high content of organic compounds, which cannot be removed by other methods [
58] Adsorption of drugs with the use of porous materials, mainly activated carbon, is known as one of the most effective processes for removing these groups of pharmaceuticals, and is thus widely used. Powdered active carbon is often used in adsorption processes [
59,
60]. It contains numerous pores of different sizes and has different functional groups on its surface. However, its disadvantage is the difficulty associated with the regeneration of the used adsorbent and the low-selective adsorption of organic adsorbents, especially at low concentrations. Activated charcoal adsorbs a wide spectrum of medicines, especially hydrophobic compounds, owing to its well-developed pore structure, large surface area, and high degree of fragmentation. On the other hand, hydrophilic drugs are inefficiently removed [
17,
18,
61]. A disadvantage encountered with the use of activated charcoal is that the working capacity of the material is significantly reduced if natural organic matter is present, as well as regeneration of the used adsorbent. Regenerative processes significantly affect the pore structure and chemical properties of functional groups in activated carbon, thereby reducing their adsorption efficiency in relation to the removed pharmaceuticals. Thermal regeneration of activated carbon can also cause carbon losses of up to 10% of its mass, which results in the need to purchase new activated carbon. As an alternative, zeolites and mesoporous silica materials can be used. These are characterized by the need for shorter contact time, lower desorption percentage, and better structural stability (which allows regeneration at high temperature) compared with activated carbon, all of which justify their use. This paper presents the general characteristics of zeolites and mesoporous silica materials and an authoritative review of data from research publications, which have not been discussed before in other studies. While individual publications contain results describing the removal efficiency of a selected pharmaceutical (belonging to one of the two groups analyzed), there is no study providing a comparative summary of removal efficiencies and conditions of the experiments conducted for several compounds from a given group and several zeolite sorbents or mesoporous materials. Therefore, efforts have been made to include in this paper the data on the efficiency of zeolites and mesoporous materials to remove the two most common groups of pharmaceuticals—antibiotics and non-steroid pharmaceuticals—from water. The paper reviews the literature on the physicochemical properties of selected zeolites (natural, synthetic, and high silica) and mesoporous silica materials—Mobil Composition of Matter (MCM-41) and Santa Barbara Amorphous (SBA-15)—and their relation to the adsorption of selected antibiotics and non-steroid pharmaceuticals. The zeolites and mesoporous silica materials described in this paper were chosen for this review because of their high availability in the market and their proven effectiveness in removing antibiotics and non-steroidal drugs from aqueous solutions. Zeolites have been shown to have the potential to be successfully used for the adsorption of sulfamethoxazole from water [
62]. The adsorption efficiency of zeolites and mesoporous silica materials was characterized taking into account their properties and the diversity of the two analyzed groups of drugs. The paper also discusses the potential possibilities and challenges related to the use of zeolites and mesoporous silica materials in water treatment. The review serves two purposes. Firstly, it allows determining the sorption capacity (described in the literature of zeolites and two mesoporous silica materials) of MCM-41 and SBA-15 in relation to the drugs dissolved in water. Additionally, it can be used to analyze their effectiveness of drug removal and potential use in wastewater treatment and groundwater remediation. Secondly, it allows determining the structural features of the analyzed adsorbent materials, which influence their adsorption of drugs from aqueous solutions. All the collected information may be of help to select materials for water treatment in the future.