The ketone bodies, especially β-hydroxybutyrate (β-HB), derive from fatty acid oxidation and alternatively serve as a fuel source for peripheral tissues including the brain, heart, and skeletal muscle. β-HB is currently considered not solely an energy substrate for maintaining metabolic homeostasis but also acts as a signaling molecule of modulating lipolysis, oxidative stress, and neuroprotection. Besides, it serves as an epigenetic regulator in terms of histone methylation, acetylation, β-hydroxybutyrylation to delay various age-related diseases. In addition, studies support endogenous β-HB administration or exogenous supplementation as effective strategies to induce a metabolic state of nutritional ketosis. The purpose of this review article is to provide an overview of β-HB metabolism and its relationship and application in age-related diseases. Future studies are needed to reveal whether β-HB has the potential to serve as adjunctive nutritional therapy for aging.
- β-hydroxybutyrate
- ketone body
- metabolism
- supplementation
- aging
- diseases
1. Introduction
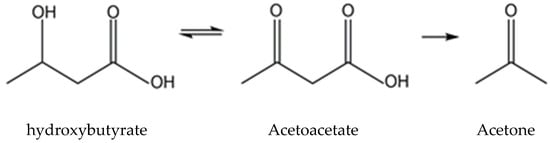
2. Overview of β-HB Metabolism
2.1. Ketone Body Production and Utilization
2.1.1. Ketogenesis in Liver
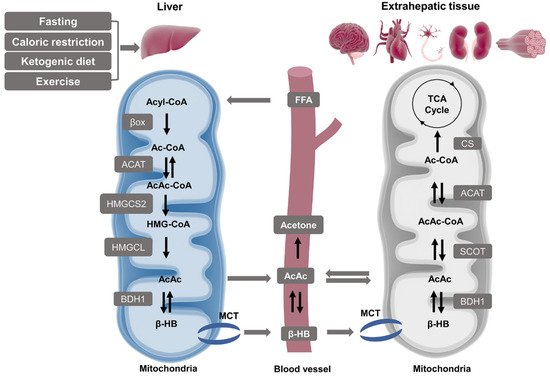
2.1.2. Ketolysis in Extra-Hepatic Tissues
2.2. β-HB as an Energy Substrate
2.3. β-HB as a Signaling Mediator
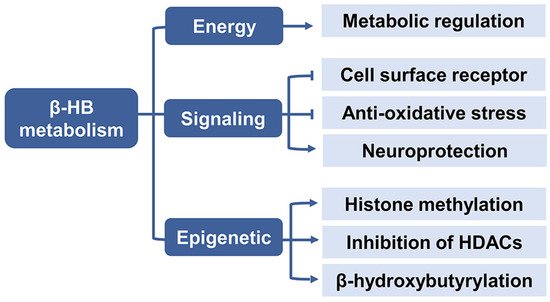
2.3.1. Cell Surface Receptor
2.3.2. Anti-Oxidative Stress
2.3.3. Neuroprotection
2.4. β-HB as an Epigenetic Regulator
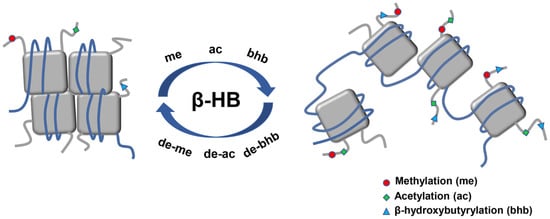
2.4.1. Histone Deacetylases Inhibition
2.4.2. Histone Lysine β-hydroxybutyrylation
3. Role of β-HB in Age-Related Diseases
3.1. Relationship between β-HB and Aging
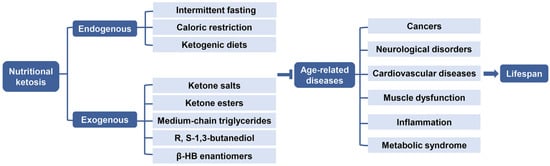
3.2. Age-Related Diseases
3.2.1. Cancers
3.2.2. Neurological Disorders
3.2.3. Cardiovascular Diseases
3.2.4. Muscle Dysfunction
3.2.5. Inflammation
3.2.6. Metabolic Syndrome
4.1. Endogenous Ketosis
Endogenous ketosis can be achieved by using various durations of fasting, CR, or CHO restriction and particularly by a KD [118](Figure 5).
4.1.1. Intermittent Fasting and Caloric Restriction
Energy-restricted metabolic states such as intermittent fasting (IF) or CR, have obvious characteristics of increased ketosis and could extend lifespan in animals [119]. Ketogenesis is believed to increase with prolonged starvation. IF is relatively easy to practice for a long time, with alternative periods of feeding or fasting which can last 24 h from one to four days per week, such as alternate-day fasting, whole-day fasting (periodic fasting), and time-restricted feeding [120,121]. IF has been proven to be safe in monitored patients and the hormonal level of this state is typically marked by a low-insulin, high-glucagon, and increased plasma fatty acids and cortisol environment which heavily promote lipolysis [122,123]. It is currently gaining more popularity and is being considered as a potential non-pharmacological way to promote healthy aging [124]. CR is achieved by reducing energy intake of about 25–30% without lacking essential nutrients, which has also been observed to improve age-related mortality and morbidity, delay aging progression, and result in healthspan in invertebrate and vertebrate species [125,126]. Circulating β-HB level is elevated as a beneficial metabolite and mediator during these two states, which are widely accepted as anti-aging interventions [127]. However, before these regimens, it is hard to maintain long-term ketosis. The mechanism of β-HB as a potential CR mimic to slow aging has yet to be explored further.
4.1.2. Ketogenic Diets
A large amount of data on the effects of β-HB metabolism comes from studies on KD, especially in rodents. KD is not an energy-limiting state, yet the related phenotype replicates some of the biochemical properties of IF and CR which are strongly associated with longevity. KD is composed of high-fat, adequate-protein, and a very low level of CHO (typically about 88%, 10%, and 2%). The KD promotes endogenous ketogenesis without fasting [128]. In terms of lifespan extension, β-HB has been proposed to promote longevity in worms via two different anti-aging pathways, which are inhibiting HDACs, leading to increased DAF-16/FOXO activity, as well as involving the mitochondrial metabolism of β-HB, and activating the SKN-1/Nrf2 antioxidant response pathway [73]. KD has also
been demonstrated to improve the longevity and survival of mice, together with increased protein acetylation and decreased activation of tissue-specific mTOR complex 1 [71]. KD has been mechanistically investigated to improve neuroprotection and mitochondrial metabolism, activate autophagy, enhance antioxidative and anti-inflammatory capability, and inhibit insulin/insulin-like growth factor signaling, which contributes to the anti-aging process [129]. Although KD has already been clinically used as a therapy, which is easier to sustain than CR, it is in some ways difficult to rigorously follow and requires specific medical guidance and strong motivation [130].
4.2. Exogenous Ketosis and Supplementation of β-HB
It has been demonstrated that not only KD, but also exogenous ketone supplements (EKSs) can increase and maintain blood KB level, especially β-HB, so as to promote anti-aging effects [131]. β-HB supplements are now being commercially marketed as an alternative to KD. The supplements are commonly present in either a powder form of ketone salts (KS) or a liquid form of ketone ester (KE). In addition, medium-chain triglycerides (MCTs) or their combination with MCTs oil are also usually used to induce and sustain NK to improve ketotic response [132,133]. The production of β-HB from these supplements would not be affected by CHO, thus administration of EKSs may be practical and alternative when maintaining a normal diet to achieve therapeutic ketogenesis (Figure 5) [134].
4.2.1. Ketone Salts
Oral administration of isolated β-HB would be the most direct method of exogenously inducing NK. However, KB in its free acid form can be expensive, unstable, and ineffective at producing sustained ketosis. Thus, ketone acids buffering with sodium, potassium, calcium, or other electrolytes have been explored to enhance efficacy, inhibit overload of any single mineral, and these compounds are commercially available. It is reported that co-ingestion with MCTs may improve the efficiency of increasing β-HB relatively, at least in rats [135]. However, a few undesirable adverse effects exist while consuming large doses of KS, which usually results in gastrointestinal distress, and inappropriate cation overload or acidosis [136].
4.2.2. Ketone Esters
Several existing synthetic KEs prove to be the most effective agents to induce immediate, sustained, and dose-dependent elevation in serum ketones concentration, which provides an alternative way to increase β-HB and is well-tolerated in rodents and humans [137,138]. Ester bonds hold KEs together and are cleaved by gastric esterases to release KB in their free acid form from the backbone molecule, which is often a ketogenic precursor molecule R,S-1,3-butanediol (BD). Two prominent KEs in the recent research studies are (R)-3-hydroxybutyl (R)-3-hydroxybutyrate ketone monoester (KME) and R,S-1,3-butanediol acetoacetate ketone diester (KDE), the former appears safer and superior at appropriate doses in healthy adults, whatever acutely or daily sustained up to 28 days [139–142]. NK produced by KEs is therefore achieved without prolonged fasting or KD yet it is currently the most potent method of EKSs.
4.2.3. Medium Chain Triglycerides
MCTs have a much greater ketogenic potential than long-chain fatty acids since they are rapidly absorbed, energy-dense, water-miscible, and tasteless. 6 to 12 carbons of fatty acids are contained in MCTs in length. MCTs can be hydrolyzed to medium-chain fatty acids by lipases in the gastrointestinal tract, and then rapidly metabolized to Ac-CoA, finally to KB in the liver [143]. MCTs are consequently regarded as ketogenic fats due to their ability of ketogenesis without the restriction of dietary CHO intake [144]. Unfortunately, high MCTs consumption are often not well adopted because of their gastrointestinal side effects, including diarrhea, dyspepsia, and flatulence, which could be alleviated through a progressive 1 or 2-week period [145]. In addition, the generation of β-HB by supplementation of MCTs is at a low level in the blood [146].
4.2.4. R, S-1,3-Butanediol
BD is an organic butyl alcohol approved by the Food and Drug Administration, which is metabolized to produce two isoforms of β-HB, D- and L-β-HB or R- and S-β-HB via hepatic conversion, even though it is not a fatty acid or MCT [147]. Oral administration of BD could achieve ketosis and approach a KD state in dogs [148]. It was demonstrated that a dose-dependent elevation of KB in a ratio of 6:1 of R-β-HB to AcAc could be produced by BD in rodents [149]. BD is often utilized as a backbone in the synthesis of KE. Gut or tissue esterases could easily break the ester bond and release KB and BD without the involvement of salts or acid [150]. A variety of preclinical toxicology studies have found that BD is safe and tolerable [151].
4.2.5. β-HB Enantiomers
β-HB is a chiral molecule, with two enantiomers, R/D and S/L, which is an important characteristic in terms of its signaling activities as well as possible therapeutic applications [152]. Currently, a racemic mixture enantiomer of β-HB is the most commercially available ingestible EKSs apart from KS and KME, as its synthesis is more affordable than pure enantiomers. The chiral specificity is introduced by BDH1, determining that only R-βHB is the normal product of human metabolism and could be readily catabolized into ATP and Ac-CoA [153]. IF, CR, KD, exercise, or any other situation which leads to endogenous β-HB would produce only R-β-HB. It is reported that ingestion of the same amount of racemic EKSs may produce higher and more sustained S-β-HB in blood circulation due to its slower metabolization compared to R-β-HB [154,155]. Despite divergent metabolic effects, these two enantiomers have similar molecular interactions as well as intracellular signal transduction cascades, which remains a hotly debated topic.
4.3. Comparisons between Endogenous and Exogenous Ketosis Induced by NK
In essence, the metabolic conditions of chronic endogenous dietary ketosis are in obvious contrast to the rapid exogenous ketosis delivered by ketone bodies. The following are the differentiations. Firstly, KD elevates blood β-HB level to a range of 0.5 mM to 3.0 mM, whereas EKSs approximately elevate to 0.3 mM to 1.0 mM [156]. Secondly, KD requires a couple of days to achieve sustained NK state, while EKSs elevate β-HB concentration acutely. Thirdly, KD need to follow strict CHO intake while EKSs require no direct CHO restriction, which determines EKSs have higher compliance, especially in short term supplementation [142]. Fourthly, circulating glucose concentrations are therefore divergent because of the diverse CHO requirement. Fifthly, their similar anabolic and
anticatabolic effects have as well been demonstrated [157,158]. Finally, KD and EKSs have been reported to reduce the substrate utilization of CHO during exercise, yet under distinct metabolic states [159,160]. NK is defined as a metabolic state, exerting physiological changes at both systemic and cellular level wherein β-HB concentration is over 0.5 mM regardless if induced by endogenous or exogenous ketones. These effects can be similar or different and can be universal or tissue-specific. NK is believed to be a potential state for performance-enhancing or therapeutic benefits under endogenous or exogenous ketosis, which needs further evaluation.
5. Future Perspectives
Future studies are necessary to further elucidate the following practical issues: activating endogenous NK in a normal dietary context to sustain a steady state of metabolism; improving the targeted delivery of β-HB prodrugs or precursors to avoid excessive salt load or acidosis; bringing β-HB to the sites of action by using existing endogenous transporters and metabolite gradients to explore specific downstream signaling events; confirming whether the synthetic KE compounds need been strictly pure due to its enormous financial burden for the majority of patients and health systems; investigating if S-β-HB has a better pharmacokinetic than R-β-HB, which might help reduce cost and discover another signaling function; establishing methodologies to quantify β-HB flux rates and differentiate these two enantiomers in terms of concentrations and impacts. Besides, the recommended dose and timing of β-HB supplements, the short half-life and bitter taste of KME as well as the interaction with other substrates in various nutritional surroundings are also needed to be specified. β-HB is emerging as vitally important regulators of metabolic health and longevity, alleviating aging phenotypes via multiple and yet unknown molecular mechanisms. By modulating lipolysis, energy expenditure, metabolic rate, insulin resistance, autophagy, feeding behavior, as well as exercise performance, β-HB might serve as a signaling biomolecule to affect cellular function and human healthspan. The evaluation of β-HB may be a crucial approach for the treatment of the aging population.
6. Conclusions
In conclusion, β-HB is the most abundant KB and plays a vital role as an energetic metabolite, a signaling molecule, as well as an epigenetic regulator, which could be used as a therapeutic agent in a range of cancers, neurodegeneration, traumatic brain disorders, cardiac diseases, muscle dysfunction, metabolic syndrome, and inflammation. Endogenous ketosis and an exogenous supplement may be promising strategies for numerous diseases. Further research is needed to investigate whether ketotherapeutics can promote healthy aging, and to figure out the specific relationship and underlying mechanisms between β-HB and the aging process, which may offer a novel way in delaying the onset and development of age-associated dysfunctions.
This entry is adapted from the peer-reviewed paper 10.3390/nu13103420
References
- A Paoli; A Rubini; Jeff S Volek; Keith Anthony Grimaldi; Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. European Journal of Clinical Nutrition 2013, 67, 789-796, 10.1038/ejcn.2013.116.
- Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate
- (ketogenic) diets
- Bhattacharya, K.; Matar, W.; Tolun, A.A.; Devanapalli, B.; Thompson, S.; Dalkeith, T.; Lichkus, K.; Tchan, M. The use of sodium DL-3-Hydroxybutyrate in severe acute neuro-metabolic compromise in patients with inherited ketone body synthetic disorders. Orphanet J. Rare Dis. 2020, 15, 53.
- Sharma, R.; Ramanathan, A. The Aging Metabolome-Biomarkers to Hub Metabolites. Proteomics 2020, 20, e1800407.
- Edwards, C.; Copes, N.; Bradshaw, P.C. D-ß-hydroxybutyrate: An anti-aging ketone body. Oncotarget 2015, 6, 3477–3478.
- Thomas, L.K.; Ittmann, M.; Cooper, C. The role of leucine in ketogenesis in starved rats. Biochem. J. 1982, 204, 399–403.
- Soto-Mota, A.; Norwitz, N.G.; Clarke, K. Why a d-β-hydroxybutyrate monoester? Biochem. Soc. Trans. 2020, 48, 51–59.
- Cahill, G.F., Jr.; Herrera, M.G.; Morgan, A.P.; Soeldner, J.S.; Steinke, J.; Levy, P.L.; Reichard, G.A., Jr.; Kipnis, D.M. Hormone-fuel interrelationships during fasting. J. Clin. Investig. 1966, 45, 1751–1769.
- Flatt, J.P. On the maximal possible rate of ketogenesis. Diabetes 1972, 21, 50–53.
- Garber, A.J.; Menzel, P.H.; Boden, G.; Owen, O.E. Hepatic ketogenesis and gluconeogenesis in humans. J. Clin. Investig. 1974, 54, 981–989.
- Reichard, G.A., Jr.; Owen, O.E.; Haff, A.C.; Paul, P.; Bortz, W.M. Ketone-body production and oxidation in fasting obese humans. J. Clin. Investig. 1974, 53, 508–515.
- Newman, J.C.; Verdin, E. β-hydroxybutyrate: Much more than a metabolite. Diabetes Res. Clin. Pr. 2014, 106, 173–181.
- Bock, H.; Fleischer, S. Preparation of a homogeneous soluble D-beta-hydroxybutyrate apodehydrogenase from mitochondria. J. Biol. Chem. 1975, 250, 5774–5781.
- Lehninger, A.L.; Sudduth, H.C.; Wise, J.B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J. Biol. Chem. 1960, 235, 2450–2455.
- Halestrap, A.P.; Wilson, M.C. The monocarboxylate transporter family--role and regulation. IUBMB Life 2012, 64, 109–119.
- Puchalska, P.; Crawford, P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017, 25, 262–284.
- Halestrap, A.P. The SLC16 gene family—Structure, role and regulation in health and disease. Mol. Asp. Med. 2013, 34, 337–349.
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 243–251.
- Fukao, T.; Song, X.Q.; Mitchell, G.A.; Yamaguchi, S.; Sukegawa, K.; Orii, T.; Kondo, N. Enzymes of ketone body utilization in human tissues: Protein and messenger RNA levels of succinyl-coenzyme A (CoA):3-ketoacid CoA transferase and mitochondrial and cytosolic acetoacetyl-CoA thiolases. Pediatr. Res. 1997, 42, 498–502.
- Orii, K.E.; Fukao, T.; Song, X.Q.; Mitchell, G.A.; Kondo, N. Liver-specific silencing of the human gene encoding succinyl-CoA: 3-ketoacid CoA transferase. Tohoku J. Exp. Med. 2008, 215, 227–236.
- Owen, O.E.; Morgan, A.P.; Kemp, H.G.; Sullivan, J.M.; Herrera, M.G.; Cahill, G.F., Jr. Brain metabolism during fasting. J. Clin. Investig. 1967, 46, 1589–1595.
- Sultan, A.M. D-3-hydroxybutyrate metabolism in the perfused rat heart. Mol. Cell Biochem. 1988, 79, 113–118.
- Abbasi, J. Ketone Body Supplementation-A Potential New Approach for Heart Disease. JAMA 2021, 326, 17–18.
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015, 6, 27.
- Krebs, H. Biochemical aspects of ketosis. Proc. R. Soc. Med. 1960, 53, 71–80.
- Robinson, A.M.; Williamson, D.H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 1980, 60, 143–187.
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426.
- Johnson, R.H.; Walton, J.L.; Krebs, H.A.; Williamson, D.H. Metabolic fuels during and after severe exercise in athletes and non-athletes. Lancet 1969, 2, 452–455.
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional ketosis for weight management and reversal of metabolic syndrome. Curr. Nutr. Rep. 2018, 7, 97–106.
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22.
- Balasse, E.O.; Féry, F. Ketone body production and disposal: Effects of fasting, diabetes, and exercise. Diabetes Metab. Rev. 1989, 5, 247–270.
- Balasse, E.O.; Fery, F.; Neef, M.A. Changes induced by exercise in rates of turnover and oxidation of ketone bodies in fasting man. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 44, 5–11.
- Rojas-Morales, P.; Tapia, E.; Pedraza-Chaverri, J. β-Hydroxybutyrate: A signaling metabolite in starvation response? Cell. Signal. 2016, 28, 917–923.
- Cotter, D.G.; Schugar, R.C.; Crawford, P.A. Ketone body metabolism and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1060–H1076.
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652.
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035.
- Offermanns, S. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharm. Sci. 2006, 27, 384–390.
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132.
- Won, Y.J.; Lu, V.B.; Puhl, H.L., 3rd; Ikeda, S.R. β-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J. Neurosci. 2013, 33, 19314–19325.
- Tunaru, S.; Kero, J.; Schaub, A.; Wufka, C.; Blaukat, A.; Pfeffer, K.; Offermanns, S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 2003, 9, 352–355.
- Lukasova, M.; Malaval, C.; Gille, A.; Kero, J.; Offermanns, S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Investig. 2011, 121, 1163–1173.
- Kovács, Z.; D’Agostino, D.P.; Diamond, D.; Kindy, M.S.; Rogers, C.; Ari, C. Therapeutic Potential of Exogenous Ketone Supplement Induced Ketosis in the Treatment of Psychiatric Disorders: Review of Current Literature. Front. Psychiatry 2019, 10, 363.
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82.
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462.
- Yang, H.; Shan, W.; Zhu, F.; Wu, J.; Wang, Q. Ketone bodies in neurological diseases: Focus on neuroprotection and underlying mechanisms. Front. Neurol. 2019, 10, 585.
- Kashiwaya, Y.; Takeshima, T.; Mori, N.; Nakashima, K.; Clarke, K.; Veech, R.L. D-beta-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 5440–5444.
- Fu, S.P.; Li, S.N.; Wang, J.F.; Li, Y.; Xie, S.S.; Xue, W.J.; Liu, H.M.; Huang, B.X.; Lv, Q.K.; Lei, L.C.; et al. BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-κB activation. Mediat. Inflamm. 2014, 2014, 983401.
- Norwitz, N.G.; Hu, M.T.; Clarke, K. The mechanisms by which the ketone body D-β-Hydroxybutyrate may improve the multiple cellular pathologies of Parkinson’s disease. Front. Nutr. 2019, 6, 63.
- Włodarek, D. Role of ketogenic diets in neurodegenerative diseases (Alzheimer’s disease and Parkinson’s disease). Nutrients 2019, 11, 169.
- Shaafi, S.; Najmi, S.; Aliasgharpour, H.; Mahmoudi, J.; Sadigh-Etemad, S.; Farhoudi, M.; Baniasadi, N. The efficacy of the ketogenic diet on motor functions in Parkinson’s disease: A rat model. Iran. J. Neurol. 2016, 15, 63–69.
- Tieu, K.; Perier, C.; Caspersen, C.; Teismann, P.; Wu, D.C.; Yan, S.D.; Naini, A.; Vila, M.; Jackson-Lewis, V.; Ramasamy, R.; et al. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J. Clin. Investig. 2003, 112, 892–901.
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398.
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52.
- Xie, Z.; Zhang, D.; Chung, D.; Tang, Z.; Huang, H.; Dai, L.; Qi, S.; Li, J.; Colak, G.; Chen, Y.; et al. Metabolic regulation of gene expression by histone lysine β-Hydroxybutyrylation. Mol. Cell 2016, 62, 194–206.
- Kontopoulos, E.; Parvin, J.D.; Feany, M.B. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 2006, 15, 3012–3023.
- Lang, C.; Campbell, K.R.; Ryan, B.J.; Carling, P.; Attar, M.; Vowles, J.; Perestenko, O.V.; Bowden, R.; Baig, F.; Kasten, M.; et al. Single-cell sequencing of iPSC-dopamine neurons reconstructs disease progression and identifies HDAC4 as a regulator of Parkinson cell phenotypes. Cell Stem Cell 2019, 24, 93–106.
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 2016, 5, e15092.
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781.
- Xu, D.; Lian, D.; Wu, J.; Liu, Y.; Zhu, M.; Sun, J.; He, D.; Li, L. Brain-derived neurotrophic factor reduces inflammation and hippocampal apoptosis in experimental Streptococcus pneumoniae meningitis. J. Neuroinflamm. 2017, 14, 156.
- Makar, T.K.; Trisler, D.; Sura, K.T.; Sultana, S.; Patel, N.; Bever, C.T. Brain derived neurotrophic factor treatment reduces inflammation and apoptosis in experimental allergic encephalomyelitis. J. Neurol. Sci. 2008, 270, 70–76.
- Mattson, M.P.; Lovell, M.A.; Furukawa, K.; Markesbery, W.R. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J. Neurochem. 1995, 65, 1740–1751.
- Kirschner, P.B.; Jenkins, B.G.; Schulz, J.B.; Finkelstein, S.P.; Matthews, R.T.; Rosen, B.R.; Beal, M.F. NGF, BDNF and NT-5, but not NT-3 protect against MPP+ toxicity and oxidative stress in neonatal animals. Brain Res. 1996, 713, 178–185.
- Zhang, X.; Cao, R.; Niu, J.; Yang, S.; Ma, H.; Zhao, S.; Li, H. Molecular basis for hierarchical histone de-β-hydroxybutyrylation by SIRT3. Cell Discov. 2019, 5, 35.
- Dąbek, A.; Wojtala, M.; Pirola, L.; Balcerczyk, A. Modulation of cellular biochemistry, epigenetics and metabolomics by ketone bodies. Implications of the ketogenic diet in the physiology of the organism and pathological states. Nutrients 2020, 12, 788.
- Boison, D. New insights into the mechanisms of the ketogenic diet. Curr. Opin. Neurol. 2017, 30, 187–192.
- Ruan, H.B.; Crawford, P.A. Ketone bodies as epigenetic modifiers. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 260–266.
- Liu, K.; Li, F.; Sun, Q.; Lin, N.; Han, H.; You, K.; Tian, F.; Mao, Z.; Li, T.; Tong, T.; et al. p53 β-hydroxybutyrylation attenuates p53 activity. Cell Death Dis. 2019, 10, 243.
- Huang, H.; Zhang, D.; Weng, Y.; Delaney, K.; Tang, Z.; Yan, C.; Qi, S.; Peng, C.; Cole, P.A.; Roeder, R.G.; et al. The regulatory enzymes and protein substrates for the lysine β-hydroxybutyrylation pathway. Sci. Adv. 2021, 7, eabe2771.
- Witte, A.V.; Fobker, M.; Gellner, R.; Knecht, S.; Flöel, A. Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. USA 2009, 106, 1255–1260.
- Roberts, M.N.; Wallace, M.A.; Tomilov, A.A.; Zhou, Z.; Marcotte, G.R.; Tran, D.; Perez, G.; Gutierrez-Casado, E.; Koike, S.; Knotts, T.A.; et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 2017, 26, 539–546.
- Veech, R.L.; Bradshaw, P.C.; Clarke, K.; Curtis, W.; Pawlosky, R.; King, M.T. Ketone bodies mimic the life span extending properties of caloric restriction. IUBMB Life 2017, 69, 305–314.
- Edwards, C.; Canfield, J.; Copes, N.; Rehan, M.; Lipps, D.; Bradshaw, P.C. D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging 2014, 6, 621–644.
- Han, Y.M.; Bedarida, T.; Ding, Y.; Somba, B.K.; Lu, Q.; Wang, Q.; Song, P.; Zou, M.H. β-Hydroxybutyrate prevents vascular senescence through hnRNP A1-mediated upregulation of Oct4. Mol. Cell 2018, 71, 1064–1078.
- Cheng, C.W.; Biton, M.; Haber, A.L.; Gunduz, N.; Eng, G.; Gaynor, L.T.; Tripathi, S.; Calibasi-Kocal, G.; Rickelt, S.; Butty, V.L.; et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell 2019, 178, 1115–1131.
- Aunan, J.R.; Cho, W.C.; Søreide, K. The biology of aging and cancer: A brief overview of shared and divergent molecular hallmarks. Aging Dis. 2017, 8, 628–642.
- Kumari, S.; Badana, A.K.; Malla, R. Reactive oxygen species: A key constituent in cancer survival. Biomark Insights 2018, 13, 1177271918755391.
- Klement, R.J.; Champ, C.E.; Otto, C.; Kämmerer, U. Anti-tumor effects of ketogenic diets in mice: A meta-analysis. PLoS ONE 2016, 11, e0155050.
- Poff, A.M.; Ari, C.; Seyfried, T.N.; D’Agostino, D.P. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS ONE 2013, 8, e65522.
- Otto, C.; Kaemmerer, U.; Illert, B.; Muehling, B.; Pfetzer, N.; Wittig, R.; Voelker, H.U.; Thiede, A.; Coy, J.F. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer 2008, 8, 122.
- Allen, B.G.; Bhatia, S.K.; Anderson, C.M.; Eichenberger-Gilmore, J.M.; Sibenaller, Z.A.; Mapuskar, K.A.; Schoenfeld, J.D.; Buatti, J.M.; Spitz, D.R.; Fath, M.A. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014, 2, 963–970.
- Klement, R.J.; Sweeney, R.A. Impact of a ketogenic diet intervention during radiotherapy on body composition: I. Initial clinical experience with six prospectively studied patients. BMC Res. Notes 2016, 9, 143.
- Schmidt, M.; Pfetzer, N.; Schwab, M.; Strauss, I.; Kämmerer, U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: A pilot trial. Nutr. Metab. 2011, 8, 54.
- Zhang, C.; Rissman, R.A.; Feng, J. Characterization of ATP alternations in an Alzheimer’s disease transgenic mouse model. J. Alzheimers Dis. 2015, 44, 375–378.
- Greenamyre, J.T.; Sherer, T.B.; Betarbet, R.; Panov, A.V. Complex I and Parkinson’s disease. IUBMB Life 2001, 52, 135–141.
- Mosconi, L.; de Leon, M.; Murray, J.; E., L.; Lu, J.; Javier, E.; McHugh, P.; Swerdlow, R.H. Reduced mitochondria cytochrome oxidase activity in adult children of mothers with Alzheimer’s disease. J. Alzheimers Dis. 2011, 27, 483–490.
- Fortier, M.; Castellano, C.A.; Croteau, E.; Langlois, F.; Bocti, C.; St-Pierre, V.; Vandenberghe, C.; Bernier, M.; Roy, M.; Descoteaux, M.; et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. 2019, 15, 625–634.
- Srivastava, S.; Baxa, U.; Niu, G.; Chen, X.; Veech, R.L. A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life 2013, 65, 58–66.
- Xu, X.; Zhang, Q.; Tu, J.; Ren, Z. D-β-hydroxybutyrate inhibits microglial activation in a cell activation model in vitro. J. Med. Coll. PLA 2011, 26, 117–127.
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269.
- Han, Y.M.; Ramprasath, T.; Zou, M.H. β-hydroxybutyrate and its metabolic effects on age-associated pathology. Exp. Mol. Med. 2020, 52, 548–555.
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192.
- Abiri, B.; Vafa, M. Dietary Restriction, Cardiovascular Aging and Age-Related Cardiovascular Diseases: A Review of the Evidence. Adv. Exp. Med. Biol. 2019, 1178, 113–127.
- Newman, J.C.; Covarrubias, A.J.; Zhao, M.; Yu, X.; Gut, P.; Ng, C.P.; Huang, Y.; Haldar, S.; Verdin, E. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab. 2017, 26, 547–557.e548.
- Shou, J.; Chen, P.J.; Xiao, W.H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14.
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model Mech. 2013, 6, 25–39.
- Stubbs, B.J.; Koutnik, A.P.; Volek, J.S.; Newman, J.C. From bedside to battlefield: Intersection of ketone body mechanisms in geroscience with military resilience. GeroScience 2021, 43, 1071–1081.
- Koutnik, A.P.; D’Agostino, D.P.; Egan, B. Anticatabolic Effects of Ketone Bodies in Skeletal Muscle. Trends Endocrinol. Metab. 2019, 30, 227–229.
- Ahola-Erkkilä, S.; Carroll, C.J.; Peltola-Mjösund, K.; Tulkki, V.; Mattila, I.; Seppänen-Laakso, T.; Oresic, M.; Tyynismaa, H.; Suomalainen, A. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum. Mol. Genet. 2010, 19, 1974–1984.
- Kwak, S.E.; Bae, J.H.; Lee, J.H.; Shin, H.E.; Zhang, D.; Cho, S.C.; Song, W. Effects of exercise-induced beta-hydroxybutyrate on muscle function and cognitive function. Physiol. Rep. 2021, 9, e14497.
- Munroe, M.; Pincu, Y.; Merritt, J.; Cobert, A.; Brander, R.; Jensen, T.; Rhodes, J.; Boppart, M.D. Impact of β-hydroxy β-methylbutyrate (HMB) on age-related functional deficits in mice. Exp. Gerontol. 2017, 87, 57–66.
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970.
- Angiolilli, C.; Baeten, D.L.; Radstake, T.R.; Reedquist, K.A. The acetyl code in rheumatoid arthritis and other rheumatic diseases. Epigenomics 2017, 9, 447–461.
- Pawelec, G.; Goldeck, D.; Derhovanessian, E. Inflammation, ageing and chronic disease. Curr. Opin. Immunol. 2014, 29, 23–28.
- Chamorro, A.; Hallenbeck, J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke 2006, 37, 291–293.
- Praticò, D.; Trojanowski, J.Q. Inflammatory hypotheses: Novel mechanisms of Alzheimer’s neurodegeneration and new therapeutic targets? Neurobiol. Aging 2000, 21, 441–453.
- Pasyukova, E.G.; Vaiserman, A.M. HDAC inhibitors: A new promising drug class in anti-aging research. Mech. Ageing Dev. 2017, 166, 6–15.
- Camberos-Luna, L.; Massieu, L. Therapeutic strategies for ketosis induction and their potential efficacy for the treatment of acute brain injury and neurodegenerative diseases. Neurochem. Int. 2020, 133, 104614.
- Kim, D.Y.; Hao, J.; Liu, R.; Turner, G.; Shi, F.D.; Rho, J.M. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS ONE 2012, 7, e35476.
- Alberti, K.G.; Zimmet, P.; Shaw, J. The metabolic syndrome--a new worldwide definition. Lancet 2005, 366, 1059–1062.
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529.
- Nunes-Souza, V.; César-Gomes, C.J.; Da Fonseca, L.J.; Guedes Gda, S.; Smaniotto, S.; Rabelo, L.A. Aging Increases Susceptibility to High Fat Diet-Induced Metabolic Syndrome in C57BL/6 Mice: Improvement in Glycemic and Lipid Profile after Antioxidant Therapy. Oxid. Med. Cell Longev. 2016, 2016, 1987960.
- Mey, J.T.; Erickson, M.L.; Axelrod, C.L.; King, W.T.; Flask, C.A.; McCullough, A.J.; Kirwan, J.P. β-Hydroxybutyrate is reduced in humans with obesity-related NAFLD and displays a dose-dependent effect on skeletal muscle mitochondrial respiration in vitro. Am. J. Physiol. Endocrinol. Metab. 2020, 319, e187–e195.
- Cavaleri, F.; Bashar, E. Potential Synergies of β-Hydroxybutyrate and Butyrate on the Modulation of Metabolism, Inflammation, Cognition, and General Health. J. Nutr. Metab. 2018, 2018, 7195760.
- Lee, A.K.; Kim, D.H.; Bang, E.; Choi, Y.J.; Chung, H.Y. β-Hydroxybutyrate Suppresses Lipid Accumulation in Aged Liver through GPR109A-mediated Signaling. Aging Dis. 2020, 11, 777–790.
- Bae, H.R.; Kim, D.H.; Park, M.H.; Lee, B.; Kim, M.J.; Lee, E.K.; Chung, K.W.; Kim, S.M.; Im, D.S.; Chung, H.Y. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget 2016, 7, 66444–66454.
- Møller, N. Ketone Body, 3-Hydroxybutyrate: Minor Metabolite—Major Medical Manifestations. J. Clin. Endocrinol. Metab. 2020, 105, dgaa370.
