Utilizing the immune system to treat cancer has been a revolutionary development and has quickly become the standard treatment for many cancer types, superseding other targeted and systemic therapies. By targeting cancer cells and avoiding the toxicities of chemotherapy and radiation, immunotherapy offers a less toxic, yet, in many types of cancers, highly efficacious alternative. With regard to PCa, the interaction between prostatic epithelial cells and the immune and non-immune cells that make up the tumor microenvironment (TME) have been shown to have an important role in the complex changes that occur and ultimately result in disease progression, development of resistant metastases, and the overall resistance to both conventional and experimental therapies.
- immunotherapy
- metastatic castration resistant prostate cancer
- tumor microenvironment
- immune resistance
- combination therapies
- immune checkpoint inhibitors
1. Introduction
Androgens have a key role in the pathogenesis of prostate cancer (PCa), and treatment modalities altering androgen receptor signaling pathways are the standard of care for advanced and disseminated disease. However, despite the initial effectiveness of androgen deprivation therapy (ADT), resistance to therapy occurs in approximately 30–50% of patients, resulting in castration-resistant prostate cancer (CRPC) for which there are very limited, generally not curative, systemic treatment options [1][2].
Utilizing the immune system to treat cancer has been a revolutionary development and has quickly become the standard treatment for many cancer types, superseding other targeted and systemic therapies [3]. By targeting cancer cells and avoiding the toxicities of chemotherapy and radiation, immunotherapy offers a less toxic, yet, in many types of cancers, highly efficacious alternative [4]. With regard to PCa, the interaction between prostatic epithelial cells and the immune and non-immune cells that make up the tumor microenvironment (TME) have been shown to have an important role in the complex changes that occur and ultimately result in disease progression, development of resistant metastases, and the overall resistance to both conventional and experimental therapies [1][5].
To date, however, patients with advanced PCa have not yet benefited to the same extent as those with more “immunologically hot” or “responsive” tumors such as melanoma, lung cancer, renal cell carcinoma or urothelial carcinoma [3][6][7]. In fact, PCa has been classified as a “cold tumor” with minimal response to immune-related treatment modalities [8][9][10]. Low tumor-associated antigen expression, decreased major histocompatibility complex (MHC) presentation of tumor antigens, tumor suppressor and DNA repair enzyme defects, and poor immune-modulating signaling are some key processes that have a role in this complex tumor environment, altering the overall anti-tumor response [8][11]. Efforts have been made to target these immune evasion mechanisms in CRPC. Currently ongoing preclinical and clinical trials are reporting encouraging results on combination-based therapies. However, other than pembrolizumab, which was approved for advanced solid tumors from the US Food and Drug Administration (FDA) in 2017 for high microsatellite instability (MSI) and in 2020 for high tumor mutational burden (TMB), sipuleucel-T, an immunotherapy based on the infusion of antigen presenting cells (APCs) which has demonstrated improvements in survival without a significant response rate, remains the only FDA-approved immunotherapy for mCRPC [12][13].
A greater understanding of the TME and methods for utilizing the host immune system to halt and eliminate tumor growth is needed to improve therapies. In this review, we set out to identify and explore key immune resistance mechanisms that lead to treatment failure in the immunosuppressive TME of PCa, and focus on therapeutic strategies ( Figure 1 ) and approaches that target the immunosuppressive TME and seek to overcome these resistance mechanisms.
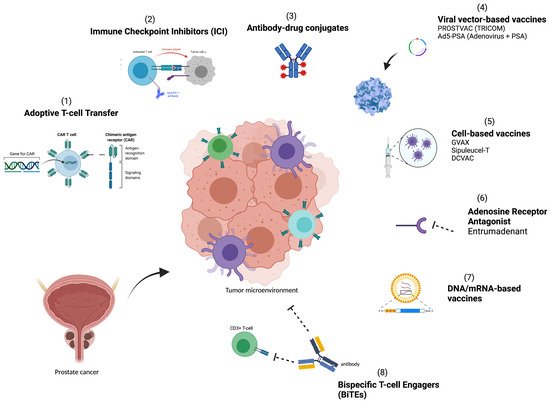
2. Immune Resistance Mechanisms in Prostate Cancer
In most solid tumors, effective immune responses within the TME rely on an increased infiltration and activation of immune cells, increased mutational burden within the cancer cells, expression of these tumor antigens on the cell surface, functional immune signaling pathways, and appropriate tumor suppressor functions [14][15]. Mechanisms that bypass these coordinated cellular functions ultimately result in immune evasion and subsequent malignant disease progression, and are thought to be critical factors in limiting the response to immune therapies [16].
Neoantigen expression on the cell surface of tumor cells via MHC Class I/Class II molecules and activation of APCs and CTLs is essential in generating an anti-tumor immune response leading to tumor cell apoptosis [17]. TMB is a quantitative measure of the total number of mutations per coding region. Because some cancers result in a higher TMB, there is a subsequent increase in tumor neoantigen expression and thus, anti-tumor immune response [17]. PCa, however, has low somatic TMB and thus, decreased neoantigen expression compared to other tumor-types [8]. In fact, one study found mean somatic mutational rates at a frequency of 0.9 per megabase, approximately 10 times lower than reported for melanoma [18]. This highlights a potential lack of T-cell co-stimulation and activation in the PCa TME, which prohibits the generation of a powerful adaptive immune response following antigen presentation; a key step in immunotherapy effectiveness [8].
Interferons (INF1) are a group of immunostimulatory cytokines released in response to cellular detection of invading pathogens [8]. Activation and expression of INF1 gene has also been shown to be crucial in mounting an efficient anti-tumor immune response through the release of cytokines that ultimately lead to an increase in the expression of immune costimulatory molecules, activation of adaptive immune cells, and an increase in TIL killing [19].
Interferon-gamma, INF-γ , a potent cytokine known to modulate tumor immunity and tumoricidal effects, has been shown to be highly elevated in patients with PCa after radiation [20]. Kundu et al. found that IFNγ can induce epithelial-to-mesenchymal transition in PCa cells leading to the downstream activation and expression of IFN-stimulated genes, PCa cell death and tumor regression [20][21]. Further animal models have also demonstrated that loss of tumor-intrinsic type I IFN can occur in proliferating PCa cells in bone and that the loss suppresses anti-tumor and therapeutic responses, in addition to promoting bone PCa cell activation and cancer progression [22]. These all highlight the importance of INF1 and their role in an anti-tumor immune response.
3. Therapies Targeting Immune Resistance
Pre-clinical studies have focused on targeted therapies against specific cancer stem cells. Cancer stem cells are a subpopulation of cancer cells with self-renewing capabilities, which are distinguished by the types of proteins expressed on their cell surface. Common cell-surface markers used in the identification of PCa stem cells include CD44, CD133, and epithelial cell adhesion molecule (EpCaM) [23].
Cancer stem cell–targeting immunotherapy has recently been attempted in PCa with chimeric antigen receptor (CAR) T cells. CAR T cells are cell-based vaccines that are a burgeoning new area of interest in immunotherapy. The precisely ex vivo engineered receptors allow the T cells to recognize and bind to specific antigens or proteins on tumor cells. Once created, the CAR T cells are engineered to express a synthetic receptor that has high affinity for specific tumor cells targeting tumor-associated antigens (TAAs) in a non-HLA complex-restricted manner [24], and are then expanded and then infused into the patient to target and kill cells carrying the specific antigen.
PCa has several overexpressed cell-surface tumor antigens, such as EpCAM, prostate stem cell antigen (PSCA) and prostate specific membrane antigen (PSMA). In one preclinical model for metastatic PCa, CAR T cells were engineered against EpCaM-expressing cancer stem cells in a murine PCa model that demonstrated inhibited tumor growth and prolonged mouse survival [25]. A preclinical model showed that PSMA-directed CAR T cells combined with docetaxel induced PCa tumor regression in a xenograft model [26]. There are several promising ongoing trials using CAR T cells to target antigens expressed in PCa including PSCA (NCT02744287), PSMA (NCT01140373), EpCAM (NCT03013712), and NY-ESO-1 (NCT03159585).
Adenosine receptors A2a and A2b are upregulated by some cancer cells and work to prevent lymphocytes and myeloid cells from infiltrating tumor cells. A2a and A2b are G protein-coupled receptors (GCPRs) that are expressed in PCa cells [27]. Etrumedenant is a new dual adenosine A2a/A2b receptor antagonist developed to target PCa. ARC-6 is an ongoing phase Ib/II open label trial evaluating etrumedenant with zimberelimab (PD-1 inhibitor) and docetaxel in patients with mCRPC (NCT04381832).
4. Combination Strategies
Due to the complex nature of immunotherapy, multifaceted combination therapies are being investigated. Trials are underway to study various permutations of treatments that include antigen vaccines, DNA vaccines, and checkpoint inhibitors. A current study is examining the effect of a neoantigen DNA vaccine in combination with PROSTVAC, nivolumab and ipilimumab (NCT03532217). Another trial is evaluating ipilimumab in combination with GVAX [28]. Pembrolizumab combination therapies in mCRPC are being investigated in KEYNOTE-365 (NCT02861573) a phase Ib/II study mentioned in the previous section with four different study medications (pembrolizumab, docetaxel, enzalutamide, Olaparib, abiraterone and prednisone). Ongoing studies are investigating combination Sipuleucel-T with atezolizumab (NCT03024216), ipilimumab (NCT01804465), radiation (NCT02463799, NCT01818986, NCT01807065), and chemotherapy (NCT01420965). A list of ongoing studies is included in Table 1 .
| Agent (s) | Mechanism | Clinical Phase | Indication | Clinical Trial ID |
|---|---|---|---|---|
| DCVAC/PCa/docetaxel/prednisone | Autologous DC vaccine + chemotherapy | III | mCRPC | NCT02111577 (VIABLE) |
| Ad/PSA | Adenovirus-based vaccine with PSA gene | II | mCRPC | NCT00583024 |
| W_pro1/cemiplimab | mRNA-based vaccine monotherapy complexed with liposomes +/− IC | I/II | mCRPC | NCT04382898 (PRO-MERIT) |
| CAR T cell-PSCA (BPX-601) | Autologous T cell-based vaccine | I/II | mCRPC | NCT02744287 |
| CAR T cell-PSMA | CAR T cell-based vaccine | I | mCRPC | NCT01140373 |
| Entrumadenant/zimberelimab/enzalutamide/docetaxel/AB680 | Adenosine receptor antagonist + ICI + CD73 inhibitor + ADT + chemotherapy | I/II | mCRPC | NCT04381832 (ARC-6) |
| AMG160/pembrolizumab | PSMA-targeting Bispecific T-cell Engager + ICI | I | mCRPC | NCT03792841 |
| HPN424 | PSMA-targeting Bispecific T-cell Engager | I/II | mCRPC | NCT03577028 |
| PROSTVAC/Ipilimumab/Nivolumab/Neoantigen DNA vaccine | Viral vector-based vaccine + ICI+ DNA vaccine | I | HSPC | NCT03532217 |
| Pembrolizumab/enzalutamide/docetaxel/olaparib/abiraterone/prednisone | ICI + ADT + PARP inhibitor + chemotherapy | I/II | mCRPC | NCT02861573 (KEYNOTE-365) |
| Atezolizumab/Sipuleucel-T | ICI + DC Vaccine | Ib | mCRPC | NCT03024216 |
| Ipilimumab/Sipuleucel-T | ICI + DC Vaccine | II | mCRPC | NCT01804465 |
| Sipuleucel-T/CT-011/Cyclophosphamide | DC Vaccine + ICI + chemotherapy | I | mCRPC | NCT01420965 |
| Nivolumab/Ipilimumab/Cabazitaxel/Prednisone | ICI + chemotherapy | II | mCRPC | NCT02985957 (CheckMate 650) |
This entry is adapted from the peer-reviewed paper 10.3390/cancers13194757
References
- Bahmad, H.F.; Jalloul, M.; Azar, J.; Moubarak, M.M.; Samad, T.A.; Mukherji, D.; Al-Sayegh, M.; Abou-Kheir, W. Tumor Microenvironment in Prostate Cancer: Toward Identification of Novel Molecular Biomarkers for Diagnosis, Prognosis, and Therapy Development. Front. Genet. 2021, 12, 652747.
- Cheng, H.H.; Lin, D.W.; Yu, E.Y. Advanced clinical states in prostate cancer. Urol. Clin. N. Am. 2012, 39, 561–571.
- Reimers, M.A.; Slane, K.E.; Pachynski, R.K. Immunotherapy in Metastatic Castration-Resistant Prostate Cancer: Past and Future Strategies for Optimization. Curr. Urol. Rep. 2019, 20, 64.
- Alatrash, G.; Jakher, H.; Stafford, P.D.; Mittendorf, E.A. Cancer immunotherapies, their safety and toxicity. Expert Opin. Drug Saf. 2013, 12, 631–645.
- Shiao, S.L.; Chu, G.C.; Chung, L.W. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 2016, 380, 340–348.
- Beer, T.M.; Kwon, E.D.; Drake, C.G.; Fizazi, K.; Logothetis, C.; Gravis, G.; Ganju, V.; Polikoff, J.; Saad, F.; Humanski, P.; et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients with Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2017, 35, 40–47.
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712.
- Vitkin, N.; Nersesian, S.; Siemens, D.R.; Koti, M. The Tumor Immune Contexture of Prostate Cancer. Front. Immunol. 2019, 10, 603.
- Nair, S.S.; Weil, R.; Dovey, Z.; Davis, A.; Tewari, A.K. The Tumor Microenvironment and Immunotherapy in Prostate and Bladder Cancer. Urol. Clin. N. Am. 2020, 47, e17–e54.
- Laccetti, A.L.; Subudhi, S.K. Immunotherapy for metastatic prostate cancer: Immuno-cold or the tip of the iceberg? Curr. Opin. Urol. 2017, 27, 566–571.
- Handa, S.; Hans, B.; Goel, S.; Bashorun, H.O.; Dovey, Z.; Tewari, A. Immunotherapy in prostate cancer: Current state and future perspectives. Ther. Adv. Urol. 2020, 12, 1756287220951404.
- Madan, R.A.; Antonarakis, E.S.; Drake, C.G.; Fong, L.; Yu, E.Y.; McNeel, D.G.; Lin, D.W.; Chang, N.N.; Sheikh, N.A.; Gulley, J.L. Putting the Pieces Together: Completing the Mechanism of Action Jigsaw for Sipuleucel-T. J. Natl. Cancer Inst. 2020, 112, 562–573.
- Wong, R.L.; Yu, E.Y. Refining Immuno-Oncology Approaches in Metastatic Prostate Cancer: Transcending Current Limitations. Curr. Treat. Options Oncol. 2021, 22, 13.
- Bander, N.H.; Yao, D.; Liu, H.; Chen, Y.T.; Steiner, M.; Zuccaro, W.; Moy, P. MHC class I and II expression in prostate carcinoma and modulation by interferon-alpha and -gamma. Prostate 1997, 33, 233–239.
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271.
- Bryant, G.; Wang, L.; Mulholland, D.J. Overcoming Oncogenic Mediated Tumor Immunity in Prostate Cancer. Int. J. Mol. Sci. 2017, 18, 1542.
- Maleki Vareki, S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J. Immunother. Cancer 2018, 6, 157.
- Berger, M.F.; Lawrence, M.S.; Demichelis, F.; Drier, Y.; Cibulskis, K.; Sivachenko, A.Y.; Sboner, A.; Esgueva, R.; Pflueger, D.; Sougnez, C.; et al. The genomic complexity of primary human prostate cancer. Nature 2011, 470, 214–220.
- Theofilopoulos, A.N.; Baccala, R.; Beutler, B.; Kono, D.H. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 2005, 23, 307–336.
- Lo, U.G.; Pong, R.C.; Yang, D.; Gandee, L.; Hernandez, E.; Dang, A.; Lin, C.J.; Santoyo, J.; Ma, S.; Sonavane, R.; et al. IFNγ-Induced IFIT5 Promotes Epithelial-to-Mesenchymal Transition in Prostate Cancer via miRNA Processing. Cancer Res. 2019, 79, 1098–1112.
- Kundu, M.; Roy, A.; Pahan, K. Selective neutralization of IL-12 p40 monomer induces death in prostate cancer cells via IL-12-IFN-γ. Proc. Natl. Acad. Sci. USA 2017, 114, 11482–11487.
- Owen, K.L.; Gearing, L.J.; Zanker, D.J.; Brockwell, N.K.; Khoo, W.H.; Roden, D.L.; Cmero, M.; Mangiola, S.; Hong, M.K.; Spurling, A.J.; et al. Prostate cancer cell-intrinsic interferon signaling regulates dormancy and metastatic outgrowth in bone. EMBO Rep. 2020, 21, e50162.
- Li, F.; Glinskii, O.V.; Mooney, B.P.; Rittenhouse-Olson, K.; Pienta, K.J.; Glinsky, V.V. Cell surface Thomsen-Friedenreich proteome profiling of metastatic prostate cancer cells reveals potential link with cancer stem cell-like phenotype. Oncotarget 2017, 8, 98598–98608.
- Bansal, D.; Reimers, M.A.; Knoche, E.M.; Pachynski, R.K. Immunotherapy and Immunotherapy Combinations in Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 334.
- Deng, Z.; Wu, Y.; Ma, W.; Zhang, S.; Zhang, Y.Q. Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM. BMC Immunol. 2015, 16, 1–9.
- Alzubi, J.; Dettmer-Monaco, V.; Kuehle, J.; Thorausch, N.; Seidl, M.; Taromi, S.; Schamel, W.; Zeiser, R.; Abken, H.; Cathomen, T.; et al. PSMA-Directed CAR T Cells Combined with Low-Dose Docetaxel Treatment Induce Tumor Regression in a Prostate Cancer Xenograft Model. Mol. Ther. Oncol. 2020, 18, 226–235.
- Sek, K.; Mølck, C.; Stewart, G.D.; Kats, L.; Darcy, P.K.; Beavis, P.A. Targeting Adenosine Receptor Signaling in Cancer Immunotherapy. Int. J. Mol. Sci 2018, 19, 3837.
- van den Eertwegh, A.J.; Versluis, J.; van den Berg, H.P.; Santegoets, S.J.; van Moorselaar, R.J.; van der Sluis, T.M.; Gall, H.E.; Harding, T.C.; Jooss, K.; Lowy, I.; et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 509–517.
