Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Conventional bone grafting procedures used to treat bone defects have several limitations. An important aspect of bone tissue engineering is developing novel bone substitute biomaterials for bone grafts to repair orthopedic defects. Considerable attention has been given to chitosan, a natural biopolymer primarily extracted from crustacean shells, which offers desirable characteristics, such as being biocompatible, biodegradable, and osteoconductive.
- chitosan
- scaffold
- biomaterials
- bone tissue engineering
- regenerative dentistry
1. Introduction
Tissue engineering by implanting artificial materials has become one of the most highly investigated scientific fields and can be used in regenerative medicine. The primary purposes of tissue engineering can be categorized as restoring, replacing, maintaining, or enhancing the function of different types of biological tissues [1,2]. Chitosan, a natural-based biopolymer, is a deacetylated form of chitin, a major by-product of crustacean shells. Currently, chitosan has attained enormous attention in several industrial applications, including biomedicine, antibacterial food coating, and the pharmaceutical and cosmetic industries [3,4]. Chitosan has received significant interest in bone regeneration [5] because it presents outstanding properties, such as being environmentally friendly, good biocompatibility, sustained drug release, biodegradable, and antimicrobial effects. Chitosan can be used in different biopolymeric composite materials [6].
2. Bone Tissue Engineering
Bone is a complex dynamic living tissue that undergoes regrowth and self-repair by modeling and remodeling processes [7]. Bone tissue is responsible for several functions in the human body, e.g., support the body structurally, withstand load bearing, protect the vital organs, and provide an environment for bone marrow [8]. However, the capacity of bone to heal a defect and restore function to an injured bone is often insufficient, especially in a large bony defect. The term tissue engineering, which combines materials science, mechanical engineering, and biology, was first introduced in 1988 [9]. BTE is a research area concerning inventing novel implantable bone substitute materials for critical-sized bone defects that cannot spontaneously heal and aiming to overcome the drawbacks of the current clinical bone disease treatments [1]. The conventional bone tissue engineering model requires three components combining osteogenic stem cells with bioactive molecules (growth factors, genes, and drugs) and seeding them onto three-dimensional (3D) biomaterial scaffolds. These scaffolds are an osteogenic implantable material that can create an ideal environment to accelerate new bone formation and induce new functional tissue integrated into the host bone without causing any adverse reaction. These three components (osteogenic stem cells, bioactive molecules, and scaffolds) are known as the tissue engineering triad.
3. BTE Scaffold
An essential aspect of BTE is developing implantable scaffolds that contribute to bone regeneration. Because the cells alone cannot grow in a 3D manner, BTE scaffolds allow the adherence of osteogenic stem cells and provide a suitable environment for the osteogenic cells to differentiate and regenerate new bone. Scaffolds are usually subdivided into three classes based on their original material base, i.e., polymer, ceramic, and metal scaffolds [10,11,12]. The Web of Science® database from 2017 to 2021 represented by 7915 articles shows that polymer scaffolds remain the most commonly developed, followed by ceramics, and are often used in polymer–ceramic combinations as a composite scaffold (Figure 1). Each scaffold type and its combinations have different advantages and disadvantages (Table 1).
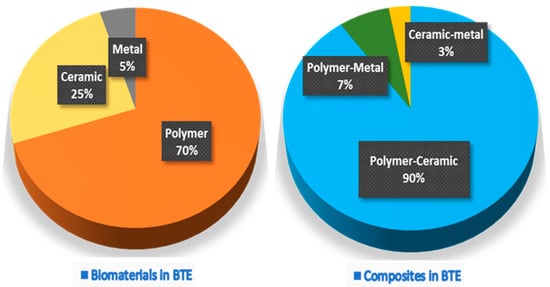
Figure 1. The proportion of pure biomaterials and their composites used in various BTE applications.
| Material Type | Advantage | Disadvantage | Example Materials |
|---|---|---|---|
| Metal | Biocompatibility Bioinert Good mechanical properties Fatigue resistance |
Bioactive molecules cannot be integrated Not biodegradable Metal ion release Low elasticity |
Titanium alloy Magnesium alloy Iron alloy |
| Ceramic | Biocompatibility Osteoinductive properties Good mechanical properties |
Low fracture toughness High brittleness Difficult to manufacture Slow degradation |
Hydroxyapatite (HA) Calcium carbonate (CC) Dicalcium phosphate (DCP) Octacalcium phosphate (OCP) β-Tricalcium phosphate (β-TCP) Biphasic calcium phosphate (BCP) |
| Polymer | Biocompatibility Low antigenicity response Easy formability Enzymatic biodegradability Easy chemical modification Crosslinking capacity |
Low osteoinductive capacity Poor mechanical properties |
Synthetic polymers Polylactic-co-glycolic acid (PLGA) Polylactic acid (PLA) Polyglycolides (PGA) Polycaprolactone (PCL) Natural polymers Collagen Cellulose Hyaluronan Fibrin Chitosan |
| Composite | Combines the advantages of each material type |
Difficult to fabricate | β-TCP-Chitosan HA-Chitosan HA-Collagen HA-PLGA |
Ideal BTE scaffolds should have specific fundamental properties (Figure 2) to use as a bone-inducing material: (1) Biocompatible, i.e., the material is compatible with living tissue and similar to the native extracellular matrix (ECM). Biocompatible scaffolds do not produce toxic by-products or induce an immune response when exposed to the body. (2) Biodegradable, i.e., the scaffold thoroughly breaks down in a predictable time, concurrent with the regeneration of new bone. (3) Strong mechanical properties to support the applied load transfer during the degradation period. (4) Interconnected porosities with pores ranging from 200 to 350 μm for successful diffusion of essential nutrients, waste transfer, and angiogenesis. (5) Controlled deliverability for releasing the appropriate dose of bioactive molecules (growth factors, genes, or drugs) directly in the desired tissue area [9,10,11,13,14].
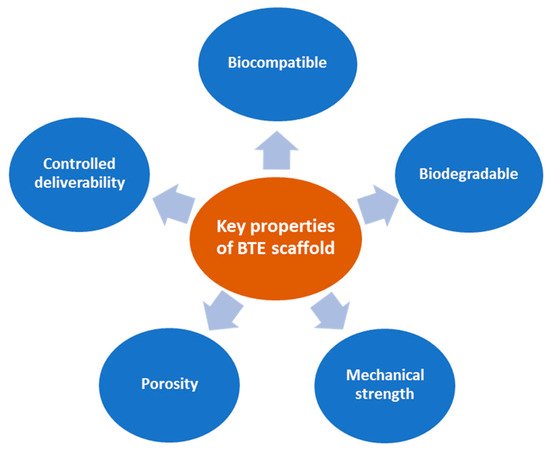
Figure 2. Fundamental properties of ideal BTE scaffolds.
4. Chitin and Chitosan
Chitin (β-(1→4)-poly-N-acetyl-D-glucosamine) is the second most abundant long-chain aminopolysaccharide polymer occurring in nature after cellulose and was first identified in mushrooms in 1811 [4,16]. Although chitin provides strength to the cell wall of some fungi, it is present in the cuticles or exoskeletons of insects, arthropods, mollusks and is mainly isolated from crustaceans, including crab, lobster, crayfish, king crab, and shrimp (Figure 3). For biomedical applications, chitin in the solid-state can be converted through enzymatic or chemical deacetylation to its most well-known fibrous substance derivative, chitosan [3].
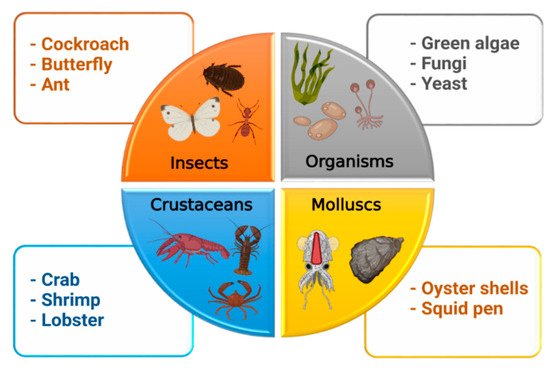
Figure 3. Sources of chitosan.
Chemically, chitosan is a semi-crystalline de-N-acetylated analog of chitin (its parent polymer), composed of two randomly distributed monomeric units, β-(1→4)-linked D-glucosamine (deacetylated unit, amino unit) and N-acetyl-D-glucosamine (acetylated unit) (Figure 4) [17]. When the β-(1→4)-linked D-glucosamine is the predominant repeating unit, and much higher than 50%, the aminopolysaccharide chain is considered chitosan [18]. The glucosamine to N-acetyl-D-glucosamine molar ratio is referred to as the degree of deacetylation (%DD), which can be determined by NMR spectroscopy, and the %DD in commercially produced chitosan ranges from 50% to 95%. Depending on the chitin source and preparation process, its molecular weight ranges from 300 to more than 1000 kilodaltons [19]. In its crystalline form, very few solid-state chitosans have acceptable solubility in water and most organic solutions above pH 7. In contrast, in acidic solvents, the protonated free amino groups on glucosamine make chitosan soluble [20,21]. Chitosan has three vital functional groups consisting of an amino group (NH2 at C-2), abundant primary hydroxyl groups (OH at C-6), and secondary hydroxyl groups (OH at C-3) [22]. These functional groups can easily generate intermolecular hydrogen bonds without disturbing its polymerization and allow modification of chitosan chain copolymerization crosslinked with other polymeric chains, which can manufacture various types of composite scaffolds and make it an attractive candidate for bone tissue repair and regeneration.
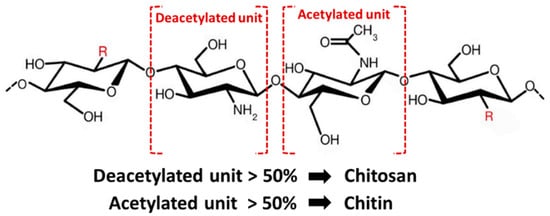
Figure 4. Structure of chitin and chitosan.
Chitosan has several essential properties, including a low-cost crustacean shells source, ease of scaffold processing, being fast and completely biodegradable, having antibacterial activity, being nonantigenic, displaying high osteoconductivity, displaying high porosity with the appropriate pore size distribution, a controlled drug delivery, and biocompatibility with almost all human tissues. These properties make chitosan attractive for a wide variety of applications, such as BTE scaffolds [3,4,17,20]. Moreover, chitosan has a chemical structure similar to glycosaminoglycans (GAG), the major component of bone′s ECM. Moreover, chitosan has become popular as a BTE scaffold because it can be easily shaped into various shapes, including 3D porous scaffolds, two-dimensional membranes/fibers, nanoparticles, and hydrogels [9,17,23,24]. Thus, chitosan scaffolds can be constructed in the shape of the bone defect.
5. Processing of Chitin and Chitosan for BTE
The most common raw material for chitin processing is crustacean shells, such as Ectes japonica (red crab), Penaeus monodon (Asian tiger shrimp), and Pandalus borealis (caridean shrimp). The industrial production of chitin from natural resources includes four steps (Figure 5) [3,23]. First, grinding the crustacean shells in a mill; second, deproteinization to remove the protein and oil in an alkaline solution at 100 °C for 4 h; third, demineralization by treating it with hydrochloric acid or sulfuric acid to remove calcium carbonate; finally, treatment with an inorganic solvent (sodium hypochlorite or hydrogen peroxide) for discoloration, washing in hot water, and grinding the particles into the appropriate size to obtain chitin powder.
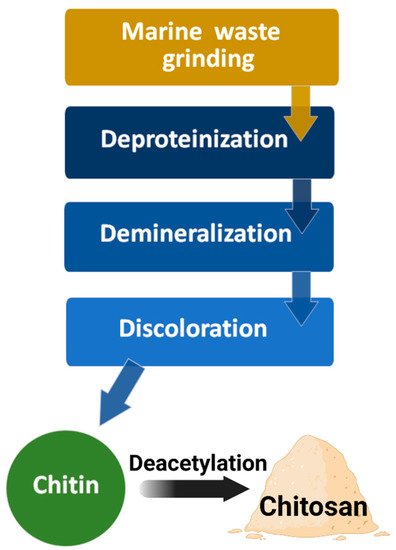
Figure 5. Diagram of chitin and chitosan processing.
In the next step of chitosan production, chitin is converted into chitosan via an enzymatic or chemical deacetylation reaction. The most common conventional technique is treating the chitin powder with a high concentration of sodium hydroxide at high temperatures (>80 °C) for 2–6 h. During this chemical deacetylation reaction, most of the acetyl groups on the long-chain polymer are removed and are converted to β-(1→4)-linked D-glucosamine (deacetylated unit, amino unit). Finally, the obtained chitosan is purified by neutralization, washing, and drying.
Several conventional techniques have been used to fabricate chitosan into a porous structure, including freeze-drying, gas foaming, solvent casting/particulate leaching (SCPL), electrospinning, and 3D-printing/rapid prototyping/bioprinting [25,26,27]. Scaffolds made from chitosan can serve as temporary structures for osteogenic cell activities and increase the new bone formation rate. Each fabrication technique has many pros and cons. The ideal fabrication technique has not yet been discovered. Several limitations need to be addressed (Table 2). The requirements of the bone defect dictate the appropriate fabrication technique or whether techniques need to be combined.
Table 2. Techniques of chitosan scaffold fabrication for BTE.
| Techniques | Description | Advantages | Disadvantages |
|---|---|---|---|
| Freeze-drying | Chitosan solutions are cooled down to a frozen state, allowed to form ice crystals followed by dehydration | Good pore interconnectivity Without high temperatures Few simple steps Easy control of porosity |
Small pore size Low porosity Long fabrication time Expensive technique |
| Gas foaming | Chitosan is placed under pressure with an inert gas, usually carbon dioxide (CO2), resulting in the nucleation of gas bubbles within the structure | Organic solvents not required Inexpensive technique |
Insufficient pore interconnectivity Insufficient mechanical strength Nonporous external surface |
| Solvent casting/particulate leaching (SCPL) | Chitosan solution is mixed with water-soluble salt particles and solidified; salt particles are then leached out | Controls the final pore size and porosity Minimal amount of material required Inexpensive technique |
Insufficient pore interconnectivity Insufficient mechanical strength -Remaining toxic porogen |
| Electrospinning | Electrostatic forces are applied to draw charged threads of chitosan solutions into fine chitosan nanofibers | Very fine fiber thickness High surface-to-volume ratio Mimics the ECM structure |
Limited cell seeding Mechanical strength and porosity decrease with fiber thickness |
| 3D-printing/ Rapid prototyping/Bioprinting |
Computer-aided design (CAD) creates a layer-by-layer 3D chitosan scaffold |
Complex 3D construct with controlled architecture and porosity Reproducible Easy incorporation of bioactive molecules |
Use of high temperatures Insufficient mechanical strength Low-throughput technology Long fabrication time |
This entry is adapted from the peer-reviewed paper 10.3390/md19100551
This entry is offline, you can click here to edit this entry!
