Cancer is one of the most extreme medical conditions in both developing and developed countries around the world, causing millions of deaths each year. Chemotherapy and/or radiotherapy are key for treatment approaches, but both have numerous adverse health effects. Furthermore, the resistance of cancerous cells to anticancer medication leads to treatment failure. The rising burden of cancer overall requires novel efficacious treatment modalities. Natural medications offer feasible alternative options against malignancy in contrast to western medication.
This review highlights the potential for furanocoumarins to be clinically beneficial in cancer, particularly given their specificity to tumor cells (while sparing normal cells). In vitro investigations have shown that furanocoumarins affect a range of cellular mechanisms, such as apoptosis, autophagy, and cell cycle arrest. ER stress induction mainly caused by NF-κB inactivation, PI3K/Akt inhibition, and p53 modulation. Furanocoumarins are also effective in different MDR cancers that are the main cause of anticancer therapeutics failure.
Compounds in this class have also have been shown to positively synergize with commonly used anticancer drugs. The fast absorption of furanocoumarins from food into the human bloodstream is also noteworthy. Furanocoumarins, by inhibiting CYP P450 3A4, not only have anticancer properties but also when co-administered with a low bioavailability anticancer compound can increase oral bioavailability. To date, most focus has been on in vitro studies, making it hard to reach solid conclusions on the efficacy of furanocoumarins in vivo.
Nonetheless, studies aimed at characterizing furanocoumarin’s efficacy in vivo as well as clinical
studies are encouraging, supporting the need for future studies to better characterize furanocoumarin’s
potential as efficacious anticancer treatment modalities.

1. Introduction
Cancer exacts one of the greatest medical tolls on humankind, requiring a proactive procedure for prevention and treatment. An enormous number of patients succumb to cancer every year. It is one of the chief reasons for mortality around the world, and the number of cases is continually expanding and estimated to reach 21 million by 2030. The lack of efficient anticancer treatments remains a clinical problem [
1,
2]. Chemotherapy and/or radiotherapy are the main clinical approaches to cancer treatment, yet both have documented adverse effects [
3,
4,
5,
6]. Cancer treatment affects not only rapidly multiplying cancerous cells but also normal body cells (bone marrow, gastrointestinal tract (GIT), and hair follicles); therefore, these treatments may give rise to severe adverse symptoms. Moreover, quick disposal and widespread distribution of the medications in cancer-free organs requires high dosing, which may lead to incremental adverse reactions. Resistance towards malignant growth is another restriction.
Restorative plants have been utilized previously. Phytopharmaceuticals primarily target malignant growth, and hence, they are the most appropriate contender for anticancer medications [
1,
2]. Nowadays, significant efforts have improved the efficacy of natural anticancer drugs with the appearance of encouraging strategies [
7,
8].
Furanocoumarins are phytochemicals that have been utilized for quite a while. The
Atharva-Veda, the Indian hallowed book, portrays the
Psoralea corylifolia poultice, and the old Egyptians utilized
Ammi majus for leukoderma (vitiligo). In 1838, 5-Methoxypsoralen (5-MOP) was the first furanocoumarin isolated from
Citrus bergamia oil by Kalbrunner [
9]. Furocoumarins are formed by coumarin and a furan ring combination, resulting in angular or linear isomers depending on the furan ring position. Angelicin and psoralen are basic furocoumarins that act as precursors for angular and linear furocoumarins, respectively. These compounds are, for the most part, biosynthesized by phenylpropanoid and the mevalonic pathways. Furocoumarins are produced in plants of Apiaceae and Rutaceae as well as in Asteraceae, Caryophyllaccae, Fabaceae, Moraceae, and Salvadoraceae for defense against insects, bacterial and fungal predators. They provide antimicrobial and insecticidal activity and behave as natural pesticides [
10]. Furocoumarins have promising therapeutic prospects, such as analgesic, anticonvulsive, anticoagulant, hypotensive, antidepressants, antibacterial, antifungal, antiviral, anti-inflammatory, antiallergic [
11,
12], antioxidants [
13], and inhibitors of human carbonic anhydrase isozymes [
14], against skin diseases [
15,
16], hyperproliferative disorders [
17,
18] and as an anticancer [
19]. This review is aimed at evaluating the literature on the anticancer potential of various furanocoumarins through different underlying mechanisms and thereof therapeutic/clinical status.
2. Chemistry of Furocoumarins
The exact molecular mechanism of such an activity relies upon the chemical structure of furanocoumarins, which depends on the furan ring and coumarin backbone combination in an angular or linear structure just as the type, location, and the number of the substituents attached [
11]. The CH3 presence at C5 improves the tumor properties of psoralen and 5-MOP, paying little heed to the substituent location. The substitution of the methoxy group with an isopentenyloxy moiety in the C5 position prompted abatement in the pro-apoptotic properties of the compound [
20,
21,
22]. Angelicin is the most straightforward angular furanocoumarin and it displays counter cancer properties. Analogous to linear furocoumarins, angular analogs can be substituted with a methoxy or isopentenyloxy group. Methoxy subordinates of angelicin incorporate isobergapten and sphondin. Isobergapten, for example, 5-methoxyangelicine, is a linear isomer of bergapten with a methoxy group joined to the fifth (C5) carbon atom. Thus, sphondin (6-methoxyangelicin) can be considered as an angular analogue of xanthotoxin. The thing that matters is, be that as it may, that the methoxy group is appended to the C6 position in 6-methoxyangelicin and to the C8 atom in the 8-MOP [
11].
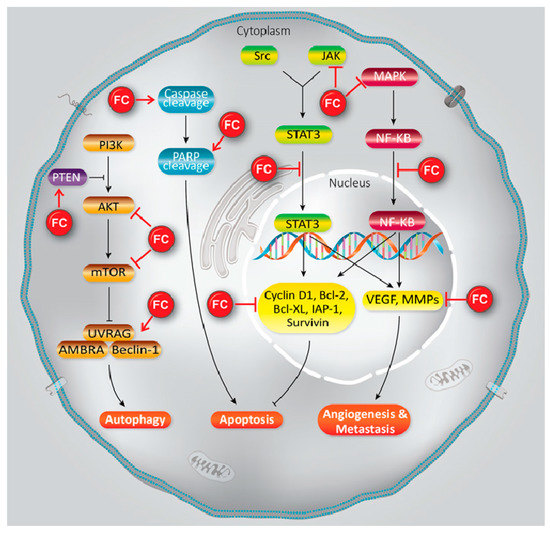
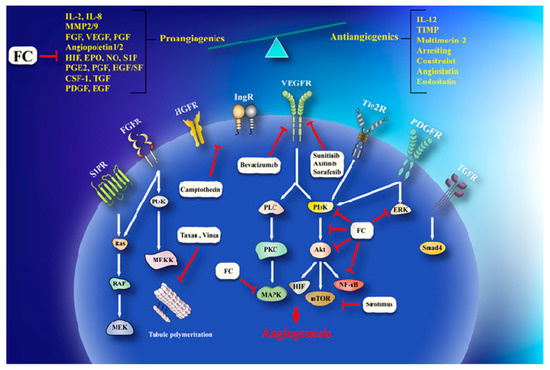
Furanocoumarins’ defensive and restorative properties have been observed in leukemia, glioma, breast, lung, renal, liver, colon, cervical, ovarian, and prostate malignancies. Apoptosis, autophagy, antioxidant, cell cycle capture, Nuclear Factor Kappa-light-chain-enhancer of activated B cells (NF-κB) inactivation, Phosphatidylinositol 3-kinase/RAC-α Serine/Threonine-Protein Kinase (PI3K/Akt) inhibition, and p53 modulation incorporate mechanistic insight (). In this article, we have reviewed the experimental data showed the role of furanocoumarins for cancer prevention and treatment.
Figure 1. A schema of different molecular mechanisms that are targeted by furanocoumarin. It shows several molecular singling pathways modulation that leads to autophagy, apoptosis, angiogenesis, and metastasis. Black lines: induce, and red lines: inhibit.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21165622