Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
Mucormycosis (also called zygomycosis) is a serious fungal infection caused by a group of molds called mucoromycetes. The types of fungi that mostly cause mucormycosis include Rhizopus spp., Mucor spp., Rhizomucor spp., Syncephalastrum spp., Cunninghamella bertholletia, Apophysomyces spp., and Lichtheimia (formerly Absidia) spp.
- severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
- mucormycosis
- covid-19
1. Pathophysiology
Previously known as zygomycosis, mucormycosis belongs to the Mucorales group of fungi [16]. The most reported causative agent of Mucorales includes the globally found Rhizopus, Mucor spp, followed by Lichtheimia spp., Rhizomucor spp., Cunninghamella spp., Apophysomyces spp., and Saksenaea spp. [20]. This opportunistic infection, present as spores or hyphae in a hot and humid environment, affects individuals if inhaled or inoculated through cutaneous wounds [21]. Immunocompromised individuals are at the greatest risk of contracting mucormycosis. Predisposing risk factors of mucormycosis include chronic kidney disease, malignancy, neutropenia, increased serum iron, perpetual use of immunosuppressive drugs, and most importantly, diabetes mellitus with ketoacidosis [22].
The immune system has macrophages and neutrophils as the primary defense system against spores. An insufficient immune response allows the spores to germinate into hyphae, and establish infection [22,23]. In diabetic individuals with ketoacidosis, an acidic pH impairs the motility of neutrophils, thus weakening the first barrier to pathogens. Iron, under normal physiologic conditions, remains bound to protein complexes; low pH renders the transferrin system inefficient as it causes unbound iron to circulate in the blood. Rhizopus has shown an increased affinity for serum iron at pH < 7.3, allowing it to multiply rapidly in the body [22,23,24]. The increased expression of the cell-surface receptor, glucose-reg-97 (GRP78) under hyperglycemic state causes R. oryzae to bind more frequently to endothelial cells in the lungs, brain, and sinuses, and thus, cause damage [25]. Virulence factors of this fungus also include aspartic proteinases which increase disease progression in individuals who have undergone organ-replacement therapy or suffer from comorbidities such as hematological malignancies [26]. Usage of corticosteroids to treat COVID-19 patients suppresses the immune system, and has emerged as a risk factor of mucormycosis, in multiple reported COVID-19 cases [27,28].
Additionally, the incidence of mucormycosis has exasperated as the deadly SARS-CoV-2 alters the immune response. SARS-CoV-2 spreads mainly through droplets and is characterized by the invasion of angiotensin-converting enzyme 2 (ACE2) receptors in humans. ACE2 is present in the lungs, heart, liver, and kidneys. Downregulation of ACE2 by the coronavirus via its spike protein negatively influences the inflammatory-protective renin-angiotensin system [29,30]. The cell-mediated immune response is rendered ineffective as CD 4+ and CD 8+ T-cells, specific for mucormycosis, decrease in COVID-19 patients. The inevitable duo of COVID-associated mucormycosis (CAM) releases a pro-inflammatory cytokine storm of IL-6 and IFN-γ in the infected individual. Lymphopenia thus paves the pathway for opportunistic fungal infections like mucor to resist immune response [29,30,31]. Fungal hyphae produce lesions and thrombi upon entering the blood vessels and invading the walls [32]. The coronavirus infection increases vascular damage to endothelial cells and promotes endotheliitis. Along with vasoconstriction, CAM possesses the capability to necrotize tissues and induce ischemia in organs leading to organ failure [31]. However, this association between the two entities has some confounding factors. For instance, it is possible that the lesions are a consequence of a Delayed-type Hypersensitivity (DTH) reaction, as for other systemic infections, such as Coccidioidomycosis. Further, it is also possible that the fungus disseminates to the skin from the primary infection site, or the cutaneous manifestations are only a consequence of fungal inoculation in the skin by a trauma. Therefore, definitive diagnostic techniques are paramount to early detection and subsequent management.
2. Clinical Manifestations
Classification of mucormycosis is based on the site of manifestation. It is enlisted as rhino-cerebral-orbital mucormycosis (RCOM), pulmonary, gastrointestinal, cutaneous, and disseminated mucormycosis, as illustrated in Table 1. RCOM is the prevalent type, and it develops in individuals with diabetic mellitus [14,33,34]. Sporangiospores deposit on the nasal turbinate, and progress through the paranasal sinuses, affecting the maxillary-facial structures, and then disseminates to the brain [35]. RCOM clinically presents as ethmoidal or sphenoidal sinusitis, and leads to cavernous sinus syndrome or internal carotid artery thrombosis. [24,36]. Osteomyelitis of the bony structures of the face results in necrotic ulcers, decreasing functional capabilities of optic and cranial nerves, causing headache and facial pain. Angioinvasion of the palate perforates it and allows the mucor infection to travel through the cribriform plate [22,37]. As it spreads to the orbital region, it degenerates the extra-ocular muscles and manifests as a periorbital syndrome, ptosis, and proptosis in patients. COVID-19 further leads to coagulopathy in the cavernous sinus which under extreme conditions progresses to a permanent loss of vision [35,38,39]. Epistaxis manifests due to the invasion of the brain by RCOM as turbinate bone, and the internal carotid artery become ischemic. Critical co-infection of COVID-19 patients with RCOM, and delayed treatment has led to increasing mortality rates [22,37].
Table 1. Summary of mucormycosis clinical manifestations in COVID-19 patients.
| Type | Pathogenesis | Clinical Manifestation | Risk Factors |
|---|---|---|---|
| Rhino-cerebral mucormycosis | Spores invade sinuses, cribriform plates, and through the cavernous sinus. | Infects the sinuses and spreads to the brain. Destroys maxillary-facial structures and causes ptosis, proptosis, and permanent vision loss [37]. | Common in patients with uncontrolled diabetes [49] and kidney transplant. |
| Pulmonary mucormycosis | Spread of fungal infection through the bloodstream. | Destroys bronchial airways, causes dyspnoea, tracheal invasions of the lungs, and a reverse halo sign on CT scan. | Patients with cancer, post-transplant immunosuppressive therapy [21]. |
| Gastrointestinal mucormycosis | Inhaling spores that invade the GI tract. | Fever, bowel, and per rectal bleed [45,50]. | Consistent use of broad-spectrum antibiotics, malnutrition, and neutropenia. |
| Cutaneous mucormycosis | Direct inoculation of skin through site of trauma or thermal burns. | Black discolouration and lesions on the skin. | Skin trauma such as surgery or burns. It does not involve an impaired immunological response. |
| Disseminated mucormycosis | Occurs when the infection spreads through the bloodstream to another part of the body | Commonly affects the brain, but also other organs such as the spleen, heart, and skin. | Iron overload, neutropenia, suppressed immune system [24]. |
Neutropenia, the use of corticosteroid, and induction chemotherapy increase the risk of pulmonary mucormycosis in individuals [24,34]. Neutropenia and a weakened immune system produce prolonged high-grade fever [24]. SARS-CoV-2 and the Rhyzomucor spp. together infect the lungs, the common site of invasion, and induce dyspnea, cough, and airway bleeding [21,37]. Molds of pulmonary mucormycosis affect bronchial airways, and the parenchyma of lungs. Extending as lesions into the chest wall, it poses the threat of cavitation and pericarditis [40]. Additionally, the characteristic and an early diagnostic feature of pulmonary mucormycosis is the reverse-halo sign which appears as consolidation on a Computed Tomography (CT) scan. However, its incidence has been low in COVID-19 patients [41]. Other than cytopenia, certain non-specific laboratory markers which have been associated with COVID-19 related sepsis are also found to be deranged in cases of co-infection with mucormycosis such as lactate dehydrogenase, C-reactive protein, and D-dimer levels, in addition to deranged renal profile [67,82].
Cutaneous mucormycosis results from trauma or burns to the skin, and mostly occurs on the arms and the legs. Surrounding edema and black discoloration at the site of infection leads to gangrene in susceptible hosts. Progressing gradually, nodular lesions appear on the skin [24,42]. While there is one reported case of coinfection in a heart transplant patient [83], COVID-19 itself is associated with vascular lesions, urticaria, and rashes [43]. This amplifies the risk of a possible cutaneous manifestation of mucormycosis. Malnutrition and solid organ transplantation put the individuals at the risk of developing gastrointestinal (GI) mucormycosis in the bowel and the GI tract, specifically the intestines [34,44]. However, the rapid disease progression and the lack of clear clinical signs besides fever delay differential diagnosis from GI diseases [38]. CT scans of isolated cases of GI tract, colonic, and small bowel mucormycosis reveal dilatation of the wall, bleeding, and mass thickening [44]. A rare case of GI mucor in COVID-19 patients confirmed the signs and additionally presented with abdominal tenderness, and bilateral ulceration [45]. Though the rarest type, disseminated mucormycosis has the highest overall mortality in individuals [38]. As a result of this, the only reported case of COVID-19 and disseminated disease was diagnosed after an autopsy [45]. The primary infection can metastasize or undergo a hematogenous spread, producing infarcts in the brain, heart, and spleen. Since it presents with symptoms of underlying comorbidities, diagnosis of disseminated mucormycosis is extremely difficult [34].
3. Geographical Distribution
Song et al. identified that critically ill patients in ICUs, and on mechanical ventilation were prone to various fungal infections, as in SARS. Other fungal infections were reported, however, until May 2020, there were no confirmed reports of mucormycosis [46].
A post-mortem study conducted between March 2020 and April 2020 from the UK revealed pathological findings in a patient, which upon biopsy, PCR, and DNA extraction confirmed the presence of disseminated mucormycosis [47]. Since then, multiple cases of mucormycosis co-infection in ongoing or post COVID-19 have emerged. Countries amidst the second and the third COVID-19 waves are now overlooking a syndemic sweeping lives globally, as shown in Figure 2. According to a review of published and unpublished studies, CAM has affected 18 countries, including but not limited to India, Pakistan France, Iran, Mexico, Russia, Bangladesh, Brazil, Chile, Czech Republic, Germany, Italy, Kuwait, Lebanon, and Turkey [41]. During the first week of June 2021, India with over 20,000 cases of CAM, remains the hardest-hit country in the world [48].
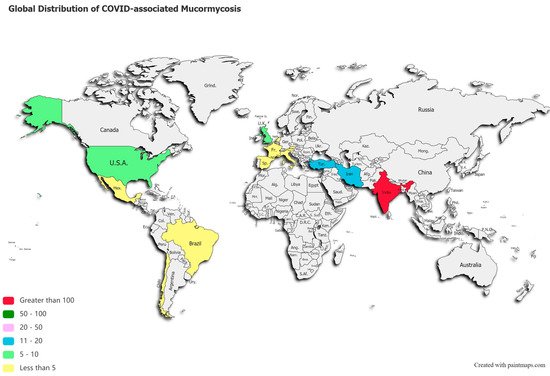
Figure 2. Global distribution of COVID-19 associated mucormycosis.
This entry is adapted from the peer-reviewed paper 10.3390/diseases9040065
This entry is offline, you can click here to edit this entry!
