Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Melatonin, a multifunctional molecule that is present in all living organisms studied, is synthesized in plant cells in several intercellular organelles including in the chloroplasts and in mitochondria.
- carbohydrates
- melatonin
- phytomelatonin
- primary metabolism
- starch
- sucrose
1. Introduction
Plants obtain their energy and resources via an autotrophic means. All their organic molecules are synthesized from inorganic elements such as CO2 and primarily from diverse compounds including nitrogen, sulfur, and phosphorus, among others. In addition to their rich secondary metabolism, plants produce a multitude of primary metabolites including carbohydrates, lipids, and amino acids. The group of carbohydrates of plant origin comprises a wide range of simple sugars such as mono- and disaccharides, sugar alcohols, and polymers such as starch and cellulose [1][2].
2. Biosynthesis of Melatonin in Plants
Melatonin (N-acetyl-5-methoxytryptamine) is a tryptophan-derived compound discovered in plants in 1995 [3][4][5]. Melatonin is a highly studied biomolecule due to its known role in mammals as a regulating hormone of sleep-wake cycles, and other functions in endogenous rhythms, mood, metabolism, and immunological responses [6][7]. In addition, it has been investigated as to its therapeutic efficacy in Alzheimer’s disease, Parkinsonism, cancer, diabetes, and SARS-CoV-2 [8][9][10][11][12][13][14].
Melatonin biosynthesis in plants originates with the amino acid tryptophan, which is endogenously synthesized in plant cells in the chorismate pathway. Five enzymes are involved in the conversion of tryptophan to melatonin; these are tryptophan decarboxylase (TDC), tryptamine 5-hydroxylase (T5H), serotonin N-acetyltransferase (SNAT), acetylserotonin methyltransferase (ASMT), and caffeic-O-methyltransferase (COMT) [15][16]. These enzymes catalyze the conversion of the indolic compounds tryptophan, tryptamine, serotonin, 5-methoxytryptamine, and N-acetylserotonin to melatonin, as illustrated in the biosynthetic pathway shown in Figure 1. However, this primary melatonin biosynthetic pathway may present alternatives such as serotonin biosynthesis through 5-hydroxytryptophan, although this possibility seems specific to animals since the responsible enzyme (tryptophan hydroxylase) has not been detected in plants. In addition, a conversion of N-acetylserotonin to serotonin by the enzyme N-acetylserotonin deacetylase has been described [15][17]. With respect to the subcellular localization, several studies in arabidopsis and rice plants indicated that the involved enzymes act in the cytoplasm (TDC, ASMT and COMT), endoplasmic reticulum (T5H), and chloroplasts (SNAT) [18]. In addition, the participation of mitochondria has been described, through arylalkylamine N-acetyltransferases (AANAT) and hydroxyindole-O-methyltransferases (HIOMT), observing that, when the melatonin pathway is artificially blocked in chloroplasts, melatonin biosynthesis shifts to the mitochondria to maintain melatonin generation [18][19]. Generally, stressors induce melatonin biosynthesis in plants through the upregulation of diverse biosynthesis isozyme transcripts, increasing endogenous melatonin production [20].
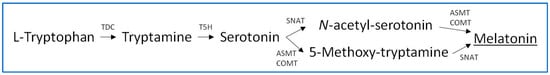
Figure 1. Biosynthesis of melatonin in plants.
3. Roles of Melatonin in Plants
Melatonin is a pleiotropic molecule in plants. Melatonin has many beneficial actions, generally improving physiological responses such as seed germination and growth, photosynthesis (pigment content, photorespiration, stomatal conductance and water economy), seed and fruit yield, osmoregulation, and the regulation of the different metabolic pathways (carbohydrates, lipids, nitrogen compounds, sulphur, and phosphorus cycles) [21][22][23][24][25][26][27][28][29][30][31]. With respect to secondary metabolism, melatonin induces the biosynthesis of simple phenols, flavonoids, anthocyanins, carotenoids, and several terpenoids [32][33][34][35]. Melatonin promotes rooting processes [36][37][38][39][40] and also delays leaf senescence [41][42][43][44][45][46]. In postharvest fruit, it regulates ethylene and lycopene content, as well as general ripening metabolism and induces parthenocarpy during fruiting [47][48][49]. It also preserves cut flowers [50][51]. In pathogen infections, melatonin slows damage, stimulating systemic acquired resistance (SAR) and contributes to crop health [52]. Due to this high number of actions, melatonin has been referred to as a plant master regulator [53][54], mainly due to its role as a plant hormone regulator, with a substantial influence on auxin, gibberellins, cytokinins, abscisic acid, ethylene, jasmonic acid, salicylic acid, and brassinosteroids [55][56].
Melatonin displays a relevant role in the stress responses. Similar to what occurs in animal cells, melatonin acts as an excellent scavenger of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in plants. This antioxidant capacity has been extensively studied [57][58][59]. The data show that melatonin acts as a direct antioxidant, neutralizing several ROS/RNS and other radical species harmful to the cell, and also acts as an activator of the antioxidant response, upregulating various transcription factors that trigger the activity of antioxidant enzymes such as superoxide dismutases, catalases, peroxidases, and those involved in the ascorbate-glutathione cycle, among others [19][60]. Via these means, melatonin acts as a master regulator of the responses of the redox, hormonal, and osmoregulatory systems [53][55][56][61]. In summary, as can be seen in Figure 2, through the redox and hormonal network, melatonin regulates photosynthesis, primary and secondary metabolism, and pathogenic response to increase abiotic/biotic tolerance and, as a result, crop yield. One of the most interesting aspects is the ability of melatonin to regulate the carbohydrate metabolism and its relationship with the osmoregulatory response, which is a key in stressful situations of plants.
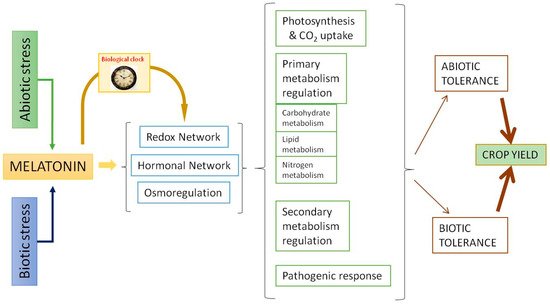
Figure 2. Melatonin actions as a response to abiotic and biotic stressors.
4. Effect of Melatonin in Simple Carbohydrates, Starch, and Polyalcohols
The term phytomelatonin refers to melatonin of plant origin as opposed to the animal hormone, but they have the identical chemical structure. The first studies on the role of phytomelatonin in plants appeared at the end of the last century and the beginning of the present one [62]. Table 1 summarizes the results of studies on melatonin and carbohydrates in plants. Based on these data, the initial report related to melatonin and carbohydrates in plants is an in vitro study in cherry rootstock. In this study, exogenous melatonin added to the culture media induced plant growth and rooting in shoot tip explants; in addition, an elevation in endogenous levels of total soluble sugars in 9-week-old plants, both in leaves and roots, and in chlorophylls, carotenoids, and proline level were also observed. These findings indicate an improvement in plant primary carbon metabolism, with a melatonin-concentration dependent response [28]. Also in apple trees, melatonin treatment of leaves produced an increase in the levels of monosaccharides, sucrose, starch, and sorbitol as well as an improvement in the photosynthetic rate and a reduction in foliar senescence and autophagy [29]. Other studies were focused on improving the plants’ tolerance to certain stresses. Thus, melatonin treatments enhanced saline tolerance in soybean [30], tomato [63], and bermudagrass plants [64] (see Table 1), accompanied by an activation of carbohydrate metabolism and, in some cases, lipid and ASC-GSH metabolism as well [65]. There are many studies on the promotional effect of fruit development after the application of melatonin in leaves and/or roots. One of the first was carried out in tomato plants, where melatonin applications induced photosynthetic processes with a higher yield in biomass and a greater number of fruits which were of greater caliber and exhibited optimal ripening [66]. In pear trees, 100 µM melatonin treatments induced higher total sugars and starch levels and better fruit sizes which were of high quality [67]. In addition, postharvest melatonin treatments in various fruits gave rise to higher quality fruits with an increased content of sugars, starch, organic acids, and pigments, as had been demonstrated in tomato [68] and banana [69], and other fruits such as peach, strawberry, pear, plum, and litchi [24][50]. In one comprehensive study, melatonin treatments induce innate immunity in Arabidopsis with the accumulation of various sugars and glycerol, as well as increasing disease resistance against Pseudomonas syringe [70]. In general, plants treated with melatonin exhibit increases in the levels of simple sugars, sucrose, starch, and some polyalcohols.
Table 1. Examples of studies on carbohydrates and melatonin.
| Plant | Melatonin Treatment (µM) | Compound Level vs. Un-Treated | Response vs. Un-Treated | Reference |
|---|---|---|---|---|
| Prunus avium x Prunus cerasus (in vitro) |
0.05–10 | ↑ total carbohydrates | ↑ rooting ↑ plant biomass |
[28] |
| Malus hupehensis tree |
100 | ↑ fructose, glucose, sucrose, starch ↑ sorbitol |
↑ photosynthesis ↓ senescence ↓ autophagy |
[29] |
| Tomato fruits | 1–500 | ↑ soluble sugars | ↑ fruit ripening and quality | [68] |
| Tomato plants | 100 | ↑ glucose, sucrose, inositol ↓ fructose, galactose |
↑ photosynthesis ↑ plant biomass ↑ fruit number and size |
[66] |
| 20–50 | ↑ soluble sugars ↑ ascorbate and GSH |
↑ photosynthesis ↑ plant growth ↑ NaCl tolerance |
[63] [71] |
|
| Soybean | 50 and 100 | ↑ carbohydrate metabolism, fatty acid biosynthesis, and ascorbate metabolism ↑ light reactions, Calvin cycle, carbohydrate, amino acid, fatty acid metabolism and Krebs cycle |
↑ germination, biomass ↑ photosynthesis ↑ cell division ↑ NaCl tolerance |
[30] |
| Bermudagrass (Cynodon dactylon) |
4–100 | 54 metabolites, including amino acids, organic acids, sugars, and sugar alcohols ↑ photosyntesis, Calvin cycle and carbohydrate metabolism |
↑ NaCl tolerance ↑ cold tolerance ↑ drought tolerance |
[64] |
| 100 | ↑ arabinose, mannose, gluco-pyranose, maltose and turanose | ↑ cold tolerance ↑ photosynthesis |
[72] | |
| Maize | 10–100 | ↑ fructose, glucose, sucrose, starch and its biosynthesis genes | ↑ photosynthesis ↑ leaf and root growth |
[73] |
| 10–1000 | ↑ total soluble sugars ↑ nitrogen compounds ↑ expressions of genes involved in C- and N- metabolisms |
↑ photosynthesis ↑ plant growth |
[74] | |
| Banana fruits | 50–500 | ↑ total soluble sugars ↑ starch |
↑ fruit ripening and quality ↓ ethylene |
[69] |
| Vicia faba | 50 | ↑ soluble sugars ↑ ascorbate and GSH |
↑ As tolerance ↑ photosynthesis ↑ plant growth |
[65] |
| Brassica juncea | 10–50 | ↑ total soluble sugars ↑ reducing sugars |
↑ photosynthesis ↑ plant growth ↑ mineral nutrition |
[75] |
| Grape plants | 50–200 | ↑ fructose, sucrose, starch, reducing sugars ↑ sucrose biosynthesis genes |
↑ photosynthesis ↑ plant growth ↑ mineral nutrition |
[76] |
| Rice plants | 20 | ↑ fructose, sucrose, starch, reducing sugars ↑ sucrose biosynthesis genes |
↑ As tolerance ↑ Krebs cycle |
[77] |
| Pear tree | 100 | ↑ total soluble sugars ↑ sucrose, starch, reducing sugars, sorbitol ↑ sucrose synthase, invertases |
↑ photosynthesis ↑ fruit size and quality |
[67] |
| Malus domestica (plants) |
1000 | ↑ fructose, glucose, sucrose, sorbitol ↓ fructokinase gene |
↑ melatonin-induced sugar accumulation ↑ growth inhibition |
[78] |
| Nicotiana tabacum (in vitro) |
0.2 | ↑ starch ↑ PEPCK and α-amylase genes |
↑ sugar starved ↑ gluconeogenesis |
[79] |
| Chinese hickory (plants) | 100 | ↑ total soluble sugars, starch ↑ proline |
↑ drought tolerance ↑ photosynthesis, transpiration |
[80] |
| Arabidopsis thaliana (Pseudomonas syringe infected) |
20 | ↑ fructose, glucose, melibose, sucrose, maltose, galatose, tagatofuranose and glycerol | ↑ bacterial innate immunity ↑ disease resistance |
[70] |
↑, Increased content or increased action; ↓, Decreased content or decreased action.
This entry is adapted from the peer-reviewed paper 10.3390/plants10091917
References
- Bryant, J.; Burrell, M.M.; Kruger, N.N. Plant Carbohydrate Biochemistry; Garland Sci./Taylor & Francis: New York, NY, USA, 1999.
- Ernst, B.; Hart, G.W.; Sinay, P. Carbohydrates in Chemistry and Biology; John Wiley & Sons: Weinheim, Germany, 2000.
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634.
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by HPLC-MS. J. Pineal Res. 1995, 18, 28–31.
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in higher plant determined by radioimmunoassay and liquid chromatography-mass spectrometry. Biol. Rhythm Res. 1995, 26, 406–409.
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Yousefi, B. The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Age Res. Rev. 2018, 47, 198–213.
- Socaciu, A.I.; Ionut, R.; Socaciu, M.A.; Ungur, A.P.; Bârsan, M.; Chiorean, A.; Socaciu, C.; Râjnoveanu, A.G. Melatonin, an ubiquitous metabolic regulator: Functions, mechanisms and effects on circadian disruption and degenerative diseases. Rev. Endocr. Metabol. Dis. 2020, 21, 465–478.
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031.
- Cardinali, D.; Brown, G.; Pandi-Perumal, S.R. Can Melatonin Be a Potential “Silver Bullet” in Treating COVID-19 Patients? Diseases 2020, 8, 44.
- Cardinali, D. Melatonin and healthy aging. Vitam. Horm. 2021, 115, 67–88.
- Talib, W.H.; Alsayed, A.R.; Abuawad, A.; Daoud, S.; Mahmod, A.I. Melatonin in Cancer Treatment: Current Knowledge and Future Opportunities. Molecules 2021, 26, 2506.
- Reiter, R.J.; Sharma, R.; Rodriguez, C.; Martin, V.; Rosales-Corral, S.; Zuccari, D.A.P.d.C.; Chuffa, L.G.d.A. Part-time cancers and role of melatonin in determining their metabolic phenotype. Life Sci. 2021, 278, 119597.
- Delpino, F.M.; Figueiredo, L.M.; Nunes, B.P. Effects of melatonin supplementation on diabetes: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2021, 40, 4595–4605.
- Pandi-Perumal, S.R.; Cardinali, D.; Reiter, R.; Brown, G. Low melatonin as a contributor to SARS-CoV-2 disease. Melatonin Res. 2020, 3, 558–576.
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437.
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Latorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40.
- Back, K. Melatonin metabolism, signaling, and possible roles in plants. Plant J. 2020, 105, 376–391.
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin synthesis and function: Evolutionary history in animals and plants. Front. Endocrinol. 2019, 10, 249.
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689.
- Ahn, H.R.; Kim, Y.J.; Lim, Y.J.; Duan, S.; Eom, S.H.; Jung, K.H. Key Genes in the Melatonin Biosynthesis Pathway with Circadian Rhythm Are Associated with Various Abiotic Stresses. Plants 2021, 10, 129.
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809.
- Arnao, M.B.; Hernández-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin against environmental plant stressors: A review. Curr. Prot. Pept. Sci. 2021, 22, 1–17.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304.
- Posmyk, M.M.; Balabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K.M. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223.
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016, 7, 718.
- Korkmaz, A.; Karakas, A.; Kocacinar, F.; Cuci, Y. The effects of seed treatment with melatonin on germination and emergence performance of pepper seeds under chilling stress. J. Agric. Sci. 2017, 23, 167–176.
- Sarropoulou, V.N.; Dimassi-Theriou, K.N.; Therios, I.N.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium x Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168.
- Wang, P.; Sun, X.; Chang, C.; Feng, F.; Liang, D.; Cheng, L.; Ma, F. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J. Pineal Res. 2013, 55, 424–434.
- Wei, W.; Li, Q.; Chu, Y.-N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707.
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295.
- Galano, A.; Castañeda-Arriaga, R.; Pérez-González, A.; Tan, D.X.; Reiter, J.R. Phenolic melatonin-related compounds: Their role as chemical protectors against oxidative stress. Molecules 2016, 21, 1442.
- Xu, L.; Yue, Q.; Bian, F.; Sun, H.; Zhai, H.; Yao, Y. Melatonin enhances phenolics accumulation partially via ethylene signaling and resulted in high antioxidant capacity in grape berries. Front. Plant Sci. 2017, 8, 1426.
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M.; Kulak, M.; Karaman, S. Seed priming with melatonin effects on growth, essential oil compounds and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress. Ind. Crops Prod. 2020, 146, 112165.
- Wang, L.; Luo, Z.; Yang, M.; Li, D.; Qi, M.; Xu, Y.; Abdelshafy, A.M.; Ban, Z.; Wang, F.; Li, L. Role of exogenous melatonin in table grapes: First evidence on contribution to the phenolics-oriented response. Food Chem. 2020, 329, 127155.
- Arnao, M.B.; Hernández-Ruiz, J. Growth activity, rooting capacity, and tropism: Three auxinic precepts fulfilled by melatonin. Acta Physiol. Plant. 2017, 39, 127.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152.
- Murch, S.J.; Campbell, S.S.B.; Saxena, P.K. The role of serotonin and melatonin in plant morphogenesis. Regulation of auxin-induced root organogenesis in in vitro-cultured explants of Hypericum perforatum L. In Vitro Cell Dev. Biol.-Plant 2001, 37, 786–793.
- Pelagio-Flores, R.; Muñoz-Parra, E.; Ortiz-Castro, R.; Lopez-Bucio, J. Melatonin regulates Arabidopsis root system architecture likely acting independently of auxin signaling. J. Pineal Res. 2012, 53, 279–288.
- Park, S.; Back, K. Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. J. Pineal Res. 2012, 53, 385–389.
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63.
- Ahmad, S.; Su, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Javed, T.; Han, Q. Foliar application of melatonin delay leaf senescence in maize by improving the antioxidant defense system and enhancing photosynthetic capacity under semi-arid regions. Protoplasma 2020, 257, 1079–1092.
- Zhao, Y.Q.; Zhang, Z.W.; Chen, Y.E.; Ding, C.B.; Yuan, S.; Reiter, R.J.; Yuan, M. Melatonin: A Potential Agent in Delaying Leaf Senescence. Crit. Rev. Plant Sci. 2021, 40, 1–22.
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 2012, 53, 11–20.
- Byeon, Y.; Park, S.; Kim, Y.S.; Park, D.H.; Lee, S.; Back, K. Light-regulated melatonin biosynthesis in rice during the senescence process in detached leaves. J. Pineal Res. 2012, 53, 107–111.
- Wang, P.; Sun, X.; Xie, Y.; Li, M.; Chen, W.; Zhang, S.; Liang, D.; Ma, F. Melatonin regulates proteomic changes during leaf senescence in Malus hupehensis. J. Pineal Res. 2014, 57, 291–307.
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharv. Biol. Technol. 2018, 139, 38–46.
- Xu, L.; Yue, Q.; Xiang, G.; Bian, F.; Yao, Y. Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Hortic. Res. 2018, 5, 41.
- Sun, Q.; Liu, L.; Zhang, L.; Lv, H.; He, Q.; Guo, L.; Zhang, X.; He, H.; Ren, S.; Zhang, N.; et al. Melatonin promotes carotenoid biosynthesis in an ethylene-dependent manner in tomato fruits. Plant Sci. 2020, 298, 110580.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in flowering, fruit set and fruit ripening. Plant Reprod. 2020, 33, 77–87.
- Murch, S.J.; Alan, A.R.; Cao, J.; Saxena, P.K. Melatonin and serotonin in flowers and fruits of Datura metel L. J. Pineal Res. 2009, 47, 277–283.
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 2020, 10, 54.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol. 2021, 23, 7–19.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin in its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207.
- Reiter, J.R.; Tan, X.D.; Rosales-Corral, S.; Galano, A.; Zhou, J.X.; Xu, B. Mitochondria: Central organelles for melatonin’s antioxidant and anti-aging actions. Molecules 2018, 23, 509.
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, 12514.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and reactive oxygen and nitrogen species: A model for the plant redox network. Melatonin Res. 2019, 2, 152–168.
- Li, D.; Wei, J.; Peng, Z.; Ma, W.; Yang, Q.; Song, Z.; Sun, W.; Yang, W.; Yuan, L.; Xu, X.; et al. Daily rhythms of phytomelatonin signaling modulate diurnal stomatal closure via regulating reactive oxygen species dynamics in Arabidopsis. J. Pineal Res. 2020, 68, e12640.
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant. 2021, 172, 820–846.
- Arnao, M.B.; Hernández-Ruiz, J. Is phytomelatonin a new plant hormone? Agronomy 2020, 10, 95.
- Siddiqui, H.M.; Alamri, S.; Al-Khaishany, Y.M.; Khan, N.M.; Al-Amri, A.; Ali, M.H.; Alaraidh, A.I.; Alsahli, A.A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353.
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694.
- Siddiqui, M.H.; Alamri, S.; Nasir Khan, M.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard. Mater. 2020, 398, 122882.
- Liu, J.; Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. The beneficial effects of exogenous melatonin on tomato fruit properties. Sci. Hortic. 2016, 207, 14–20.
- Liu, J.; Yue, R.; Si, M.; Wu, M.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Effects of exogenous application of melatonin on quality and sugar metabolism in Zaosu pear fruit. J. Plant Growth Regul. 2019, 38, 1161–1169.
- Sun, Q.Q.; Zhang, N.; Wang, J.; Zhang, H.J.; Li, D.B.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668.
- Hu, W.; Yang, H.; Tie, W.; Yan, Y.; Ding, Z.; Liu, Y.; Wu, C.; Wang, J.; Reiter, R.J.; Tan, D.X.; et al. Natural Variation in Banana Varieties Highlights the Role of Melatonin in Postharvest Ripening and Quality. J. Agric. Food Chem. 2017, 65, 9987–9994.
- Qian, Y.; Tan, D.X.; Reiter, R.J.; Shi, H. Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis. Sci. Rep. 2015, 5, 15815.
- Ali, M.; Kamran, M.; Abbasi, G.H.; Saleem, M.H.; Ahmad, S.; Parveen, A.; Malik, Z.; Afzal, S.; Ahmar, S.; Dawar, K.M.; et al. Melatonin-Induced Salinity Tolerance by Ameliorating Osmotic and Oxidative Stress in the Seedlings of Two Tomato (Solanum lycopersicum L.) Cultivars. J. Plant Growth Regul. 2020.
- Fan, J.; Hu, Z.; Xie, Y.; Chan, Z.; Chen, K.; Amombo, E.; Chen, L.; Fu, J. Alleviation of cold damage to photosystem II and metabolisms by melatonin in Bermudagrass. Front. Plant Sci. 2015, 6, 925.
- Zhao, H.; Su, T.; Huo, L.; Wei, H.; Jiang, Y.; Xu, L.; Ma, F. Unveiling the mechanism of melatonin impacts on maize seedling growth: Sugar metabolism as a case. J. Pineal Res. 2015, 59, 255–266.
- Erdal, S. Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Rep. 2019, 38, 1001–1012.
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Melatonin modulates photosynthesis, redox status, and elemental composition to promote growth of Brassica juncea: A dose-dependent effect. Protoplasma 2020, 257, 1685–1700.
- Zhong, L.; Lin, L.; Yang, L.; Liao, M.; Wang, X.; Wang, J.; Lv, X.; Deng, H.; Liang, D.; Xia, H.; et al. Exogenous melatonin promotes growth and sucrose metabolism of grape seedlings. PLoS ONE 2020, 15, e0232033.
- Samanta, S.; Singh, A.; Banerjee, A.; Roychoudhury, A. Exogenous supplementation of melatonin alters representative organic acids and enzymes of respiratory cycle as well as sugar metabolism during arsenic stress in two contrasting indica rice cultivars. J. Biotech. 2020, 324, 220–232.
- Yang, J.; Zhang, C.; Wang, Z.; Sun, S.; Zhan, R.; Zhao, Y.; Ma, B.; Ma, F.; Li, M. Melatonin-Mediated Sugar Accumulation and Growth Inhibition in Apple Plants Involves Down-Regulation of Fructokinase 2 Expression and Activity. Front. Plant Sci. 2019, 10, 150.
- Kobylinska, A.; Borek, S.; Posmyk, M.M. Melatonin redirects carbohydrates metabolism during sugar starvation in plant cells. J. Pineal Res. 2018, 64, e12466.
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci. Total Environ. 2020, 713, 136675.
This entry is offline, you can click here to edit this entry!
