Liver cancer is among the leading global healthcare issues associated with high morbidity and mortality. Liver cancer consists of hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), hepatoblastoma (HB), and several other rare tumors. Progression has been witnessed in understanding the interactions between etiological as well as environmental factors and the host in the development of liver cancers. However, the pathogenesis remains poorly understood, hampering the design of rational strategies aiding in preventing liver cancers. Accumulating evidence demonstrates that aberrant activation of the Wnt/β-catenin signaling pathway plays an important role in the initiation and progression of HCC, CCA, and HB. Targeting Wnt/β-catenin signaling potentiates a novel avenue for liver cancer treatment, which may benefit from the development of numerous small-molecule inhibitors and biologic agents in this field.
- liver cancer
- precancerous lesion
- HCC
- CCA
- HB
- Wnt/β-catenin signaling
1. Introduction
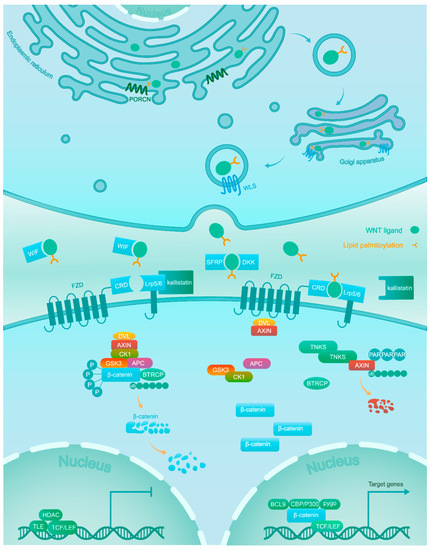
2. Wnt/β-Catenin Signaling
This entry is adapted from the peer-reviewed paper 10.3390/cancers11070926
References
- Farazi, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687.
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179.
- Darbari, A.; Sabin, K.M.; Shapiro, C.N.; Schwarz, K.B. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology 2003, 38, 560–566.
- Aretz, S.; Koch, A.; Uhlhaas, S.; Friedl, W.; Propping, P.; von Schweinitz, D.; Pietsch, T. Should children at risk for familial adenomatous polyposis be screened for hepatoblastoma and children with apparently sporadic hepatoblastoma be screened for APC germline mutations? Pediatr. Blood Cancer 2006, 47, 811–818.
- Qu, B.; Liu, B.R.; Du, Y.J.; Chen, J.; Cheng, Y.Q.; Xu, W.; Wang, X.H. Wnt/β-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol. Lett. 2014, 7, 1175–1178.
- Anastas, J.N.; Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 2012, 13, 11–26.
- Klaus, A.; Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 2008, 8, 387–398.
- Wang, W.; Pan, Q.; Fuhler, G.M.; Smits, R.; Peppelenbosch, M.P. Action and function of Wnt/beta-catenin signaling in the progression from chronic hepatitis C to hepatocellular carcinoma. J. Gastroenterol. 2017, 52, 419–431.
- Peifer, M.; Polakis, P. Wnt signaling in oncogenesis and embryogenesis—A look outside the nucleus. Science 2000, 287, 1606–1609.
- Dahmani, R.; Just, P.A.; Perret, C. The Wnt/beta-catenin pathway as a therapeutic target in human hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 709–713.
- Liu, X.; Zhang, B.; McBride, J.D.; Zhou, K.; Lee, K.; Zhou, Y.; Liu, Z.; Ma, J.X. Antiangiogenic and antineuroinflammatory effects of kallistatin through interactions with the canonical Wnt pathway. Diabetes 2013, 62, 4228–4238.
- Hart, M.; Concordet, J.P.; Lassot, I.; Albert, I.; del los Santos, R.; Durand, H.; Perret, C.; Rubinfeld, B.; Margottin, F.; Benarous, R.; et al. The F-box protein beta-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr. Biol. 1999, 9, 207–210.
- Zhong, Y.; Katavolos, P.; Nguyen, T.; Lau, T.; Boggs, J.; Sambrone, A.; Kan, D.; Merchant, M.; Harstad, E.; Diaz, D.; et al. Tankyrase Inhibition Causes Reversible Intestinal Toxicity in Mice with a Therapeutic Index <1. Toxicol. Pathol. 2016, 44, 267–278.
- Mariotti, L.; Pollock, K.; Guettler, S. Regulation of Wnt/beta-catenin signalling by tankyrase-dependent poly(ADP-ribosyl)ation and scaffolding. Br. J. Pharm. 2017, 174, 4611–4636.
- Tian, Y.; Mok, M.T.; Yang, P.; Cheng, A.S. Epigenetic Activation of Wnt/beta-Catenin Signaling in NAFLD-Associated Hepatocarcinogenesis. Cancers (Basel) 2016, 8, 76.
- Sharma, M.; Jamieson, C.; Johnson, M.; Molloy, M.P.; Henderson, B.R. Specific armadillo repeat sequences facilitate beta-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358. J. Biol. Chem. 2012, 287, 819–831.
- Ma, L.; Wei, W.; Chua, M.-S.; So, S. WNT/β-catenin pathway activation in hepatocellular carcinoma: A clinical perspective. Gastrointest. Cancer Targets Ther. 2014, 4, 49–63.
