Hepatitis E virus (HEV), a pathogen that causes acute viral hepatitis, is a small icosahedral, quasi-enveloped, positive ssRNA virus. Its genome has three open reading frames (ORFs), with ORF1 and ORF3 encoding for nonstructural and regulatory proteins, respectively, while ORF2 is translated into the structural, capsid protein. ORF2 is most widely used for vaccine development in viral hepatitis. Hepatitis E virus-like particles (VLPs) are potential vaccine candidates against HEV infection. VLPs are composed of capsid subunits mimicking the natural configuration of the native virus but lack the genetic material needed for replication. As a result, VLPs are unable to replicate and cause disease, constituting safe vaccine platforms. Currently, the recombinant VLP-based vaccine Hecolin® against HEV is only licensed in China.
- Hepatitis E virus
- ORF2 capsid protein
- HEV VLPs
- vaccine
1. Introduction
Hepatitis E virus (HEV) is an enterically transmitted pathogen and a major cause of acute hepatitis in many developing countries within Africa and Asia [1]. Approximately one third of the world population live in areas in which HEV is endemic and thus are at risk of infection [2]. Unlike other viruses causing hepatitis, HEV-related disease is a zoonotic infection with pigs, wild boars and certain other species such as deer and rabbits being considered as reservoirs for the virus [3][4]. Although the fatality rate during epidemics is low, i.e., between 0.2–5% [5], the mortality rate in pregnant women is as high as 25%, possibly due to altered hormone status and decreased immunity [6][7][8]. Even though HEV infection is considered self-limiting or asymptomatic in healthy individuals, it can lead to severe disease in patients with preexisting liver conditions, with high morbidity and mortality [9][10]. Chronic infection could develop in immunocompromised patients such as organ transplant recipients [11], individuals administered immunosuppressants [12], patients on chemotherapy for hematological malignancies [13], HIV-infected patients [14] and cases of superinfection with other hepatitis viruses [15]. In 10% of chronically infected patients, HEV leads to rapid progression to liver cirrhosis in less than 3 years [16]. In addition, it has become evident in recent years that HEV infections can be associated with neurological manifestations [17][18], renal aliments [19], hematological disorders [20] and acute pancreatitis [21]. Furthermore, recent data indicate a link between HEV infection and progression to hepatocellular carcinoma in patients infected with hepatitis B virus (HBV) or hepatitis C virus (HCV) [22][23]. Atsama et al. [22] reported significantly higher prevalence of anti-HEV IgG in hepatocellular carcinoma (HCC) patients infected with either HBV or HCV compared with HBV/HCV-infected patients with chronic liver disease but not suffering from HCC [22]. This finding suggests that infection with HEV could worsen liver inflammation and increase the severity of other infections. Another study also reported that HEV superinfection accelerates the progression of chronic HBV infection and increases 1-year mortality [23].
Traditional approaches for the development of an HEV vaccine have been ruled out because the manufacturing of either live attenuated or inactivated vaccine would be impossible due to the complexity and low yield of viral culture. Even though culturing the virus has been difficult in the past, a few strains have been adapted to cell culture, leading to a better understanding of the HEV life cycle [24].
Presently, significant progress has been made in the development of HEV vaccines based on the ORF2 capsid protein as either a subunit or virus-like particle (VLP) [25]. VPLs represent one of the most attractive systems for vaccine development due to their safety, immunogenic properties and ease of production [26]. VLPs are generated from one or more viral capsid proteins that self-assemble into high-molecular-weight structures that resemble the native virions but lack the viral genome [27]. As a result, VLPs are replication- and infection-incompetent, making them a safe alternative to attenuated or inactivated viruses in vaccine development. Since they are structurally similar to the native virus, they can induce stronger B and T cell responses than traditional small subunit vaccines [28]. Additionally, VLPs can be better taken up by professional antigen-presenting cells (APCs) as exogenous and endogenous antigens for processing and presentation by MHC class II and I molecules, respectively. Cross-presentation by MHC class molecules activates CD4+ and CD8+ T cells that elicit specific cytotoxic T lymphocyte (CTL) responses resulting in infection control [29]. Furthermore, VLPs can be assembled not only from proteins from a single virus, but also from proteins of distinct viruses or various other pathogens, e.g., bacteria and protozoa [30]. To date, several VLPs have been produced for protection against infectious diseases in prokaryotic or eukaryotic expression systems [31], and in some cases assembled in cell-free conditions [32]. Some of these products have been licensed, including Engerix® (Hepatitis B virus) [33], Cervarix® (human papilloma virus) [34], Recombivax HB® (HBV) [35] and Gardasil® (HPV) [36], while others are still under pre-clinical and clinical evaluation [37][38].
2. Hepatitis E Genome Organization
Previously known as non-A non B hepatitis, HEV is currently classified in the Hepeviridae family with the two genera Orthohepeviruses and Pischihepeviruses [39]. The Orthohepevirus A genus includes genotypes 1 and 2 isolated from humans, genotypes 3 and 4 from both humans and animals, the newly proposed genotypes 5 and 6 from wild boars and genotype 7 from dromedary camels [40][41].
HEV is a quasi-enveloped, icosahedral, single-stranded positive-sense RNA virus that was molecularly characterized for the first time in 1990 [42]. Its genome is around 7.2 kb with features of a eukaryotic mRNA, including a 5′ cap and 3′ poly A tail, 5′ and 3′ untranslated regions (UTRs), and three open reading frames, including ORF1, ORF2, and ORF3 [43]. During HEV genome replication two viral RNA species are generated, i.e., the full-length genomic RNA and a subgenomic RNA [44]. The subgenomic RNA allows the expression of ORF2 and ORF3 (Figure 1).
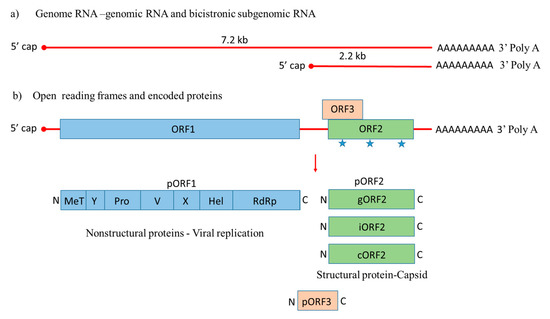
Figure 1. Genome organization of Hepatitis E virus. (a) Hepatitis E-Virus (HEV) genome generates the full-length genomic RNA and subgenomic RNA with 5′ cap, 3′ Poly A tail, 5′ UTR and 3′ UTR. (b) The genomic RNA has three open reading frames: ORF1, ORF2, and ORF3. ORF1 encodes the nonstructural proteins for viral replication; ORF2 is translated into the capsid protein with three potential glycosylation sites ( ), with a small multifunctional protein encoded by ORF3. Three different capsid proteins have been discovered in vitro during infection, i.e., gORF2-glycosylated, iORF2-infectious and cORF2-cleaved ORF2.
), with a small multifunctional protein encoded by ORF3. Three different capsid proteins have been discovered in vitro during infection, i.e., gORF2-glycosylated, iORF2-infectious and cORF2-cleaved ORF2.
ORF1 encodes nonstructural proteins involved in viral replication [45][46]. A small multifunctional 13 kDa protein is expressed from ORF3, which facilitates HEV transport throughout the cell and acts as viroporin for the release of the infectious virus from the host cell [47][48]. ORF2 encodes the 72 kDa capsid protein comprising 660 amino acids that contains a hydrophobic stretch of 14–34 amino acids at the N-terminus, which functions as a signal sequence for its secretion [49]. ORF2 is involved in virion assembly, attachment to the host cell and immunogenicity [50][51][52]. Additionally, the capsid protein has three potential glycosylation sites (Asn 132, 310 and 562) [53].
Native HEV particles are round non-enveloped with spikes covering the surface [54][55]. It is considered that 180 copies of the ORF2 protein form the HEV virion giving it T = 3 icosahedral symmetry [56]. Recently, a few strains have been adapted for replication in cell culture, providing novel insights into the HEV cycle. Even though HEV particles present in the bile and feces are non-enveloped, it was demonstrated that in patient serum and cell cultures, HEV particles are partially associated with lipids and the ORF3 protein [57]. Moreover, recent studies have identified different forms of ORF2 in cultured cells. Large ORF2 protein amounts are released from HEV-infected cells in vitro and found in serum from HEV-infected patients. This secreted protein (ORF2s) was shown to be glycosylated form of the capsid protein that is not associated with the HEV virion. The other intracellular protein (ORF2c), a translation product of the same gene starting with the second AUG codon, is involved in HEV assembly [58]. Montpellier et al. reported iORF2 (infectious), gORF2 (glycosylated) and additional ORF2 truncated protein (ORF2c) are not involved in virion assembly using another genotype and cell culture for replication [59].
Great efforts have been made towards understanding the HEV life cycle in recent years by developing cellular systems and infectious HEV clones [60]. Polarized cell models have been developed to closely mimic in vivo infection with HEV, which are highly permissive to infection, making them a good tool for molecular studies of the HEV cycle. For example, human hepatoma-derived HepaRG and porcine hepatocyte-like PICM-19 cell lines have been shown to support HEV replication, and are useful for studying virus–host interactions and species barrier crossing, especially since HEV infection is a zoonosis in developed countries [61]. Capelli et al. [62][63] showed that different HEV genotypes release more than 90% of the virus from the apical membrane after infecting polarized human hepatocellular carcinoma HepG2/C3A cells, suggesting the main route of release for infectious virions [62][63]. In recent years, the key steps of HEV’s natural infectious cycle in vivo have been confirmed by employing polarized human stem-cell-derived, hepatocyte-like cells (HLCs). Infection of these cells with HEV results in the secretion of two different progeny particle types, including quasi-enveloped particles from the basolateral membrane and naked highly infectious virions from the apical membrane [64]. These findings provide novel insights into the HEV infectious cycle. The release of HEV particles basolaterally could spread the infection in the host and lead to extrahepatic manifestations [65].
This entry is adapted from the peer-reviewed paper 10.3390/v12080826
References
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.-S.; Ijaz, S.; Izopet, J.; Dalton, H.R. Hepatitis E. Lancet 2012, 379, 2477–2488.
- Holla, R.P.; Ahmad, I.; Ahmad, Z.; Jameel, S. Molecular virology of hepatitis E virus. In Seminars in Liver Disease; Thieme Medical Publishers, New York, NY, USA : 2013; pp. 3–14.
- Meng, X.-J. From barnyard to food table: The omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011, 161, 23–30.
- Kumar, S.; Subhadra, S.; Singh, B.; Panda, B. Hepatitis E virus: The current scenario. Int. J. Infect. Dis. 2013, 17, e228–e233.
- Panda, S.K.; Thakral, D.; Rehman, S. Hepatitis E virus. Rev. Med Virol. 2007, 17, 151–180.
- Khuroo, M.S.; Teli, M.R.; Skidmore, S.; Sofi, M.A.; Khuroo, M.I. Incidence and severity of viral hepatitis in pregnancy. Am. J. Med. 1981, 70, 252–255.
- Khuroo, M.; Kamili, S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J. Viral Hepat. 2003, 10, 61–69.
- Jilani, N.; Das, B.C.; Husain, S.A.; Baweja, U.K.; Chattopadhya, D.; Gupta, R.K.; Sardana, S.; Kar, P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J. Gastroenterol. Hepatol. 2007, 22, 676–682.
- Allaire, M.; Bazille, C.; Selves, J.; Salamé, E.; Altieri, M. Hepatitis E virus infection mimicking acute graft rejection in a liver transplant recipient. Clin. Res. Hepatol. Gastroenterol. 2018, 42, e68–e71.
- Behrendt, P.; Steinmann, E.; Manns, M.P.; Wedemeyer, H. The impact of hepatitis E in the liver transplant setting. J. Hepatol. 2014, 61, 1418–1429.
- Marion, O.; Kamar, N. Hepatitis E Infections in Transplants. Emerg. Transpl. Infect. Clin. Chall. Implic. 2020, 1–18, doi:10.1007/978-3-030-01751-4_36-1.
- Pischke, S.; Peron, J.-M.; von Wulffen, M.; von Felden, J.; Höner zu Siederdissen, C.; Fournier, S.; Lütgehetmann, M.; Iking-Konert, C.; Bettinger, D.; Thimme, R. Chronic hepatitis e in rheumatology and internal medicine patients: A retrospective multicenter european cohort study. Viruses 2019, 11, 186.
- Tavitian, S.; Peron, J.-M.; Huguet, F.; Kamar, N.; Abravanel, F.; Beyne-Rauzy, O.; Oberic, L.; Faguer, S.; Alric, L.; Roussel, M. Ribavirin for chronic hepatitis prevention among patients with hematologic malignancies. Emerg. Infect. Dis. 2015, 21, 1466.
- Rivero-Juarez, A.; Lopez-Lopez, P.; Frias, M.; Rivero, A. Hepatitis E infection in HIV-infected patients. Front. Microbiol. 2019, 10, 1425.
- Kumar, M.; Sharma, B.C.; Sarin, S.K. Hepatitis E virus as an etiology of acute exacerbation of previously unrecognized asymptomatic patients with hepatitis B virus‐related chronic liver disease. J. Gastroenterol. Hepatol. 2008, 23, 883–887.
- Gérolami, R.; Moal, V.; Colson, P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N. Engl. J. Med. 2008, 358, 859–860.
- Mclean, B.N.; Gulliver, J.; Dalton, H.R. Hepatitis E virus and neurological disorders. Pract. Neurol. 2017, 17, 282–288.
- Abravanel, F.; Pique, J.; Couturier, E.; Nicot, F.; Dimeglio, C.; Lhomme, S.; Chiabrando, J.; Saune, K.; Péron, J.-M.; Kamar, N. Acute hepatitis E in French patients and neurological manifestations. J. Infect. 2018, 77, 220–226.
- Kamar, N.; Weclawiak, H.; Guilbeau-Frugier, C.; Legrand-Abravanel, F.; Cointault, O.; Ribes, D.; Esposito, L.; Cardeau-Desangles, I.; Guitard, J.; Sallusto, F. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation 2012, 93, 617–623.
- Leaf, R.K.; O‘Brien, K.L.; Leaf, D.E.; Drews, R.E. Autoimmune hemolytic anemia in a young man with acute hepatitis E infection. Am. J. Hematol. 2017, 92, E77–E79.
- Jaroszewicz, J.; Flisiak, R.; Kalinowska, A.; Wierzbicka, I.; Prokopowicz, D. Acute hepatitis E complicated by acute pancreatitis: A case report and literature review. Pancreas 2005, 30, 382–384.
- Atsama, M.A.; Atangana, P.J.A.; Noah, D.N.; Moundipa, P.F.; Pineau, P.; Njouom, R. Hepatitis E virus infection as a promoting factor for hepatocellular carcinoma in Cameroon: Preliminary observations. Int. J. Infect. Dis. 2017, 64, 4–8.
- Tseng, T.-C.; Liu, C.-J.; Chang, C.T.; Su, T.-H.; Yang, W.-T.; Tsai, C.-H.; Chen, C.-L.; Yang, H.-C.; Liu, C.-H.; Chen, P.-J. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J. Hepatol. 2020, 72, 1105–1111.
- Shukla, P.; Nguyen, H.; Faulk, K.; Mather, K.; Torian, U.; Engle, R.; Emerson, S. Adaptation of a genotype 3 hepatitis E virus to efficient growth in cell culture depended on an inserted human gene segment acquired by recombination. J. Virol. 2012, 86, 5697–5707.
- Schofield, D.; Glamann, J.; Emerson, S.; Purcell, R. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J. Virol. 2000, 74, 5548–5555.
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176.
- Noad, R.; Roy, P. Virus-like particles as immunogens. Trends Microbiol. 2003, 11, 438–444.
- Grgacic, E.V.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65.
- Cox, R.G.; Erickson, J.J.; Hastings, A.K.; Becker, J.C.; Johnson, M.; Craven, R.E.; Tollefson, S.J.; Boyd, K.L.; Williams, J.V. Human metapneumovirus virus-like particles induce protective B and T cell responses in a mouse model. J. Virol. 2014, 88, 6368–6379.
- Rts, S.; Agnandji, S.T.; Lell, B.; Fernandes, J.F.; Abossolo, B.P.; Methogo, B.; Kabwende, A.L.; Adegnika, A.A.; Mordmueller, B.; Issifou, S. A phase 3 trial of RTS, S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 2012, 367, 2284–2295.
- Scotti, N.; Rybicki, E.P. Virus-like particles produced in plants as potential vaccines. Expert Rev. Vaccines 2013, 12, 211–224.
- Almeida, J.; Edwards, D.C.; Brand, C.; Heath, T. Formation of virosomes from influenza subunits and liposomes. Lancet 1975, 306, 899–901.
- Keating, G.M.; Noble, S. Recombinant hepatitis B vaccine (Engerix-B®). Drugs 2003, 63, 1021–1051.
- Monie, A.; Hung, C.-F.; Roden, R.; Wu, T.C. Cervarix™: A vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biol. Targets Ther. 2008, 2, 107.
- Venters, C.; Graham, W.; Cassidy, W. Recombivax-HB: Perspectives past, present and future. Expert Rev. Vaccines 2004, 3, 119–129.
- Shi, L.; Sings, H.; Bryan, J.; Wang, B.; Wang, Y.; Mach, H.; Kosinski, M.; Washabaugh, M.; Sitrin, R.; Barr, E. GARDASIL®: Prophylactic human papillomavirus vaccine development–from bench top to bed‐side. Clin. Pharmacol. Ther. 2007, 81, 259–264.
- Crevar, C.J.; Ross, T.M. Elicitation of protective immune responses using a bivalent H5N1 VLP vaccine. Virol. J. 2008, 5, 1.
- Treanor, J.J.; Atmar, R.L.; Frey, S.E.; Gormley, R.; Chen, W.H.; Ferreira, J.; Goodwin, R.; Borkowski, A.; Clemens, R.; Mendelman, P.M. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate—Reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J. Infect. Dis. 2014, 210, 1763–1771.
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.-J.; Okamoto, H.; Van der Poel, W.H.; Purdy, M.A.; Members of the International Committee on the Taxonomy of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232.
- Takahashi, M.; Nishizawa, T.; Nagashima, S.; Jirintai, S.; Kawakami, M.; Sonoda, Y.; Suzuki, T.; Yamamoto, S.; Shigemoto, K.; Ashida, K. Molecular characterization of a novel hepatitis E virus (HEV) strain obtained from a wild boar in Japan that is highly divergent from the previously recognized HEV strains. Virus Res. 2014, 180, 59–69.
- Woo, P.; Lau, S.; Teng, J.; Tsang, A.; Joseph, M.; Wong, E.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 2014, 20, e8.
- Reyes, G.R.; Purdy, M.A.; Kim, J.P.; Ka-Cheung, L.; Young, L.M. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 1990, 247, 1335.
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.-C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131.
- Graff, J.; Torian, U.; Nguyen, H.; Emerson, S.U. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 2006, 80, 5919–5926.
- Ahmad, I.; Holla, R.P.; Jameel, S. Molecular virology of hepatitis E virus. Virus Res. 2011, 161, 47–58.
- Cao, D.; Meng, X.-J. Molecular biology and replication of hepatitis E virus. Emerg. Microbes Infect. 2012, 1, e17.
- Kannan, H.; Fan, S.; Patel, D.; Bossis, I.; Zhang, Y.-J. The hepatitis E virus open reading frame 3 product interacts with microtubules and interferes with their dynamics. J. Virol. 2009, 83, 6375–6382.
- Ding, Q.; Heller, B.; Capuccino, J.M.; Song, B.; Nimgaonkar, I.; Hrebikova, G.; Contreras, J.E.; Ploss, A. Hepatitis E virus ORF3 is a functional ion channel required for release of infectious particles. Proc. Natl. Acad. Sci. USA 2017, 114, 1147–1152.
- Jameel, S.; Zafrullah, M.; Ozdener, M.H.; Panda, S.K. Expression in animal cells and characterization of the hepatitis E virus structural proteins. J. Virol. 1996, 70, 207–216.
- He, S.; Miao, J.; Zheng, Z.; Wu, T.; Xie, M.; Tang, M.; Zhang, J.; Ng, M.-H.; Xia, N. Putative receptor-binding sites of hepatitis E virus. J. Gen. Virol. 2008, 89, 245–249.
- Kalia, M.; Chandra, V.; Rahman, S.A.; Sehgal, D.; Jameel, S. Heparan sulfate proteoglycans are required for cellular binding of the hepatitis E virus ORF2 capsid protein and for viral infection. J. Virol. 2009, 83, 12714–12724.
- Xing, L.; Wang, J.C.; Li, T.-C.; Yasutomi, Y.; Lara, J.; Khudyakov, Y.; Schofield, D.; Emerson, S.U.; Purcell, R.H.; Takeda, N. Spatial configuration of hepatitis E virus antigenic domain. J. Virol. 2011, 85, 1117–1124.
- Torresi, J.; Li, F.; Locarnini, S.A.; Anderson, D.A. Only the non-glycosylated fraction of hepatitis E virus capsid (open reading frame 2) protein is stable in mammalian cells. J. Gen. Virol. 1999, 80, 1185–1188.
- Xing, L.; Kato, K.; Li, T.; Takeda, N.; Miyamura, T.; Hammar, L.; Cheng, R.H. Recombinant hepatitis E capsid protein self-assembles into a dual-domain T= 1 particle presenting native virus epitopes. Virology 1999, 265, 35–45.
- Guu, T.S.; Liu, Z.; Ye, Q.; Mata, D.A.; Li, K.; Yin, C.; Zhang, J.; Tao, Y.J. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc. Natl. Acad. Sci. USA 2009, 106, 12992–12997.
- Xing, L.; Li, T.-C.; Mayazaki, N.; Simon, M.N.; Wall, J.S.; Moore, M.; Wang, C.-Y.; Takeda, N.; Wakita, T.; Miyamura, T. Structure of hepatitis E virion-sized particle reveals an RNA-dependent viral assembly pathway. J. Biol. Chem. 2010, 285, 33175–33183.
- Qi, Y.; Zhang, F.; Zhang, L.; Harrison, T.J.; Huang, W.; Zhao, C.; Kong, W.; Jiang, C.; Wang, Y. Hepatitis E virus produced from cell culture has a lipid envelope. PLoS ONE 2015, 10, e0132503.
- Yin, X.; Ying, D.; Lhomme, S.; Tang, Z.; Walker, C.M.; Xia, N.; Zheng, Z.; Feng, Z. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc. Natl. Acad. Sci. USA 2018, 115, 4773–4778.
- Montpellier, C.; Wychowski, C.; Sayed, I.M.; Meunier, J.-C.; Saliou, J.-M.; Ankavay, M.; Bull, A.; Pillez, A.; Abravanel, F.; Helle, F. Hepatitis E virus lifecycle and identification of 3 forms of the ORF2 capsid protein. Gastroenterology 2018, 154, 211–223.
- Fu, R.M.; Decker, C.C.; Dao Thi, V.L. Cell culture models for hepatitis E virus. Viruses 2019, 11, 608.
- Rogee, S.; Talbot, N.; Caperna, T.; Bouquet, J.M.; Barnaud, E.; Pavio, N. New models of hepatitis E virus replication in human and porcine hepatocyte cell lines. J. Gen. Virol. 2013, 94, 549–558.
- Capelli, N.; Marion, O.; Dubois, M.; Allart, S.; Bertrand-Michel, J.; Lhomme, S.; Abravanel, F.; Izopet, J.; Chapuy-Regaud, S. Vectorial release of hepatitis E virus in polarized human hepatocytes. J. Virol. 2019, 93, 93.
- Capelli, N.; Dubois, M.; Pucelle, M.; Da Silva, I.; Lhomme, S.; Abravanel, F.; Chapuy-Regaud, S.; Izopet, J. Optimized hepatitis E virus (HEV) culture and its application to measurements of HEV infectivity. Viruses 2020, 12, 139.
- Thi, V.L.D.; Wu, X.; Belote, R.L.; Andreo, U.; Takacs, C.N.; Fernandez, J.P.; Vale-Silva, L.A.; Prallet, S.; Decker, C.C.; Fu, R.M. Stem cell-derived polarized hepatocytes. Nat. Commun. 2020, 11, 1–13.
- Kamar, N.; Marion, O.; Abravanel, F.; Izopet, J.; Dalton, H.R. Extrahepatic manifestations of hepatitis E virus. Liver Int. 2016, 36, 467–472.
