Chlorinated aliphatic hydrocarbons (CAHs) are ubiquitous contaminants whose presence in groundwater has persisted for many decades, mainly due to the physical-chemical characteristics of these compounds. In particular, CAHs belong to Dense Non-Aqueous Phase Liquids (DNAPLs), for which contamination scenarios differ significantly from Light NAPLs scenarios, where the separate phase floats at the top of the water table due to its lower density than water.
- bioremediation
- biological reductive dechlorination
- chlorinated aliphatic hydrocarbons
- sustainable materials
- biochar
- polyhydroxyalkanoates
- bioelectrochemical systems
1. Background
Chlorinated aliphatic hydrocarbons (CAHs) are ubiquitous contaminants whose presence in groundwater has persisted for many decades, mainly due to the physical-chemical characteristics of these compounds [1][2]. In particular, CAHs belong to Dense Non-Aqueous Phase Liquids (DNAPLs), for which contamination scenarios differ significantly from Light NAPLs scenarios, where the separate phase floats at the top of the water table due to its lower density than water [3][4].
The two major problems associated with aged CAHs-contaminated sites are the secondary source management and the containment and remediation of the contamination plume. Secondary source refers to the presence of the solvent in a separate phase, primarily accumulated in the low-permeability layers of the aquifer (clay lens), which work as a source of long-term contaminants [5]. On the other hand, contamination plume refers to prolonged contamination in the same direction of water flow due to the slow and steady release of secondary sources. The distribution of contaminants is highly dependent on the hydrogeological characteristics of the site and concentration levels differing greatly from source to source.
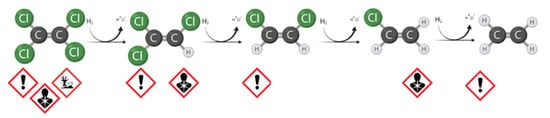
Today, in-situ technologies can operate and significantly reduce costs, although detailed site characterization is required. In this context, many improvements have been made in modeling and interpreting the data to support the design and adoption of the right strategy [6]. For the remediation of chlorinated solvents plume, the In-Situ Bioremediation (ISB) technologies are growing attention. The reasons for the success are the public support, success on the dissolved contaminants, and a comparatively low cost when it is effective [7]. If the presence of degradation intermediates is detected during site characterization, the biological Natural Attenuation phenomenon occurs due to the presence of specific microorganisms [8]. For the reductive dechlorination (RD) of widespread contaminants, such as tetrachloroethane (TeCA), perchloroethylene (PCE), and trichloroethylene (TCE), in an anaerobic environment, one of the most widely used approaches is enhanced natural attenuation (ENA) [9][10]. The intervention provides the addition of an organic fermentable substrate to produce short-chain fatty acids (acetate and other volatile fatty acids, VFAs) and the direct electron donor, the molecular hydrogen [11][12]. Therefore, almost any fermentable substrate can be a potential source of carbon and hydrogen to stimulate RD, including carbohydrates (sugars), alcohols, oils, solids (e.g., bark mulch, chitin), and complex compounds (e.g., whey and cellulose) [13]. The RD is a well-known, step-by-step reaction. Focusing on the sequential RD pathway of PCE through TCE, cis-dichloroethyene (cis-DCE), and vinil chloride (VC) to ethene, shown in Figure 1 , the efficiency of each step can be dramatically different depending on the environmental conditions and the microbial populations responsible for the reactions [14][15]. If the specific population has developed in the aquifer ( Dehalococcoides spp.) , the reaction may proceed until ethene production. As with every technology, serious issues may be associated with ISB, and the following situation may seriously affect meeting the remediation goals: Low efficacy on separate phase or highly adsorbed fraction (at source); Substrate availability limitations; and Incomplete degradation pathways (stall at an intermediate stage) for energetic and kinetic constraints with volatile and more toxic by-product accumulation, e.g., VC.
Recently, the combined approach has been investigated to help address the RD limits. Complete management of the site can be achieved more effectively with a combination of physicochemical processes to prompt anaerobic biodegradation [16]. For example, over the last few decades, the feasibility of DNAPLs’ bioremediation, also in the source areas, has been recognized through the combination of ISB with other more aggressive technologies, such as ISCO-R (In-Situ Chemical Oxidation Reduction), thermal treatment, and excavation [17]. In addition, the coupled technology is expected to be more satisfactory than a single-technology approach to deal with site changes and response during the intervention. Compared to individual methods, coupling technologies into sequences, combinations, or treatment trains can be more effective in reducing the contamination level and in the overall duration. As concerning the specific RD reactions, it is well known that the anaerobic condition favors the degradation of the highly chlorinated compounds, whereas aerobic condition favors the oxidation of di- and mono-substituted compounds [14]. For this reason, the sequential combination of technologies that might be considered opposing (reduction/oxidation) may be more effective to avoid VC accumulation.
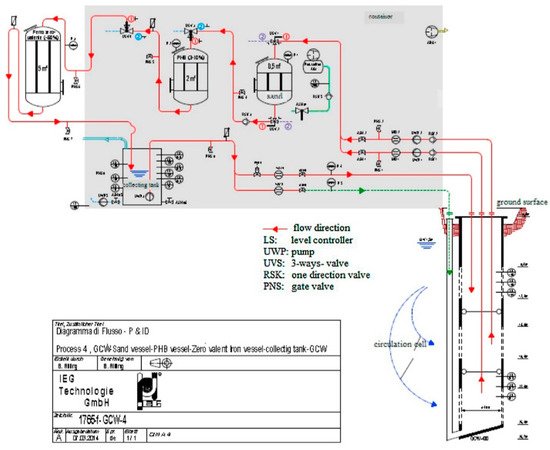
2. Combination of GCW (Groundwater Circulation Well) and ENA (Enhanced Natural Attenuation)
3. Combination of Adsorption and Biodegradation
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering8080109
References
- Weatherill, J.J.; Atashgahi, S.; Schneidewind, U.; Krause, S.; Ullah, S.; Cassidy, N.; Rivett, M. Natural attenuation of chlorinated ethenes in hyporheic zones: A review of key biogeochemical processes and in-situ transformation potential. Water Res. 2018, 128, 362–382.
- Suchomel, E.J.; Kavanaugh, M.C.; Mercer; J.W.; Johnson, P.C. The source zone remediation challenge. In Chlorinated Solvent Source Zone Remediation; Kueper, B.H., Stroo, H.F., Vogel, C.M., Ward, C.H., Eds.; Springer: New York, NY, USA, 2014; Volume 7, pp. 29–62.
- Wisconsin Department of Natural Resourcers. Understanding Chlorinated Hydrocarbon Behavior in Groundwater: Guidance on the Investigation, Assessment and Limitations of Monitored Natural Attenuation; Wisconsin Department of Natural Resources: Madison, WI, USA, 2014.
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526.
- Rodrigues, R.; Betelu, S.; Colombano, S.; Tzedakis, T.; Masselot, G.; Ignatiadis, I. In situ chemical reduction of chlorinated organic compounds. In Applied Environmental Science and Engineering for a Sustainable Future; van Hullebusch, E.D., Huguenot, D., Pechaud, Y., Simonnot, M.-O., Colombano, S., Eds.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 283–398.
- Ciampi, P.; Esposito, C.; Cassiani, G.; Petrangeli Papini, M. A 3D Multi-Source Conceptual Model to Support the Remediation of a Jet Fuel Contaminated Site. In Proceedings of the EGU General Assembly 2020, Online, 4–8 May 2020; EGU2020-9880. 2020.
- Maier, R.; Gentry, T.J. Microorganisms and organic pollutants. In Environmental Microbiology; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 377–413.
- Ciampi, P.; Esposito, C.; Petrangeli Papini, M. Hydrogeochemical model supporting the remediation strategy of a highly contaminated industrial site. Water 2019, 11, 1371.
- Rosansky, S.; Condit, W.; Sirabian, R. Best Practices for Injection and Distribution of Amendments; Technical Report TR-NAVFAC-EXWC-EV-1303; Battelle Memorial Institute and NAVFAC Alternative Restoration Technology Team: Port Hueneme, CA, USA, 2013.
- Majone, M.; Verdini, R.; Aulenta, F.; Rossetti, S.; Tandoi, V.; Kalogerakis, N.; Agathos, S.; Puig, S.; Zanaroli, G.; Fava, F. In situ groundwater and sediment bioremediation: Barriers and perspectives at European contaminated sites. New Biotechnol. 2015, 32, 133–146.
- Li, J.; Hu, A.; Bai, S.; Yang, X.; Sun, Q.; Liao, X.; Yu, C.-P. Characterization and performance of lactate-feeding consortia for reductive dechlorination of trichloroethene. Microorganisms 2021, 9, 751.
- DiStefano, T.D.; Gossett, J.M.; Zinder, S.H. Hydrogen as an electron donor for dechlorination of tetrachloroethene by an anaerobic mixed culture. Appl. Environ. Microbiol. 1992, 58, 3622–3629.
- Leeson, A.; Beevar, E.; Henry, B.; Fortenberry, J.; Coyle, C. Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents; Technical Report TR-2250-ENV; Engineering Service Center: Port Hueneme, CA, USA, 2004.
- Field, J.; Sierra-Alvarez, R. Biodegradability of chlorinated solvents and related chlorinated aliphatic compounds. Rev. Environ. Sci. Bio/Technol. 2004, 3, 185–254.
- Xiao, Z.; Jiang, W.; Chen, D.; Xu, Y. Bioremediation of typical chlorinated hydrocarbons by microbial reductive dechlorination and its key players: A review. Ecotoxicol. Environ. Saf. 2020, 202, 110925.
- Ebrahimbabaie, P.; Pichtel, J. Biotechnology and nanotechnology for remediation of chlorinated volatile organic compounds: Current perspectives. Environ. Sci. Pollut. Res. 2021, 28, 7710–7741.
- The Interstate Technology & Regulatory Council. Technical/Regulatory Guidance Integrated DNAPL Site Strategy; Technology Interstate Technology & Regulatory Council: Washington, DC, USA, 2011.
- Petrangeli, M.P.; Majone, M.; Arjmand, F.; Silvestri, D.; Sagliaschi, M.; Sucato, S.; Alesi, E. First pilot test on the integration of GCW (groundwater circulation well) with ENA (enhanced natural attenuation) for chlorinated solvents source remediation. Chem. Eng. Trans. 2016, 49, 91–96.
- Pierro, L.; Matturro, B.; Rossetti, S.; Sagliaschi, M.; Sucato, S.; Bartsch, E.; Alesi, E.; Majone, M.; Arjmand, F.; Petrangeli Papini, M. Un nuovo processo per la bonifica di sorgenti residuali di dnapl: Risultati della prima sperimen-tazione in piena scala. Ing. dell’Ambiente 2016, 3, 160–172.
- Ciampi, P.; Esposito, C.; Bartsch, E.; Alesi, E.J.; Petrangeli Papini, M. 3D dynamic model empowering the knowledge of the decontamination mechanisms and controlling the complex remediation strategy of a contaminated industrial site Science of the Total Environment 3D dynamic model empowering the knowledge of the decontaminatio. Sci. Total Environ. 2021, 793, 148649.
- Pierro, L.; Matturro, B.; Rossetti, S.; Sagliaschi, M.; Sucato, S.; Alesi, E.; Bartsch, E.; Arjmand, F.; Petrangeli Papini, M. Polyhydroxyalkanoate as a slow-release carbon source for in situ bioremediation of contaminated aquifers: From laboratory investigation to pilot-scale testing in the field. New Biotechnol. 2017, 37, 60–68.
- Matturro, B.; Pierro, L.; Frascadore, E.; Petrangeli, P.-M.; Rossetti, S. Microbial community changes in a chlorinated solvents polluted aquifer over the field scale treatment with poly-3-hydroxybutyrate as amendment. Front. Microbiol. 2018, 9, 1664.
- Hug, L.A.; Maphosa, F.; Leys, D.; Löffler, F.E.; Smidt, H.; Edwards, E.; Adrian, L. Overview of organohalide-respiring bacteria and a proposal for a classification system for reductive dehalogenases. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120322.
- O’Connor, D.; Hou, D.; Ok, Y.S.; Song, Y.; Sarmah, A.K.; Li, X.; Tack, F.M. Sustainable in situ remediation of recalcitrant organic pollutants in groundwater with controlled release materials: A review. J. Control. Release 2018, 283, 200–213.
- Aulenta, F.; Fuoco, M.; Canosa, A.; Petrangeli Papini, M.; Majone, M. Use of poly-β-hydroxy-butyrate as a slow-release electron donor for the microbial reductive dechlorination of TCE. Water Sci. Technol. 2008, 57, 921–925.
- Baric, M.; Majone, M.; Beccari, M.; Petrangeli Papini, M. Coupling of polyhydroxybutyrate (PHB) and zero valent iron (ZVI) for enhanced treatment of chlorinated ethanes in permeable reactive barriers (PRBs). Chem. Eng. J. 2012, 195, 22–30.
- Ciampi, P.; Esposito, C.; Viotti, P.; Boaga, J.; Cassiani, G.; Petrangeli Papini, M. An integrated approach supporting remediation of an aquifer contaminated with chlorinated solvents by a combination of adsorption and biodegradation. Appl. Sci. 2019, 9, 4318.
- Siggins, A.; Thorn, C.; Healy, M.G.; Abram, F. Simultaneous adsorption and biodegradation of trichloroethylene occurs in a biochar packed column treating contaminated landfill leachate. J. Hazard. Mater. 2021, 403, 123676.
- Wu, Y.; Tatsumoto, H.; Aikawa, M. Modeling of tetrachloroethylene degradation by anaerobic granular biological activated carbon. J. Health Sci. 2000, 46, 434–440.
- Islam, S.; Zhang, Y.; McPhedran, K.; Liu, Y.; El-Din, M.G. Granular activated carbon for simultaneous adsorption and biodegradation of toxic oil sands process-affected water organic compounds. J. Environ. Manag. 2015, 152, 49–57.
- Piai, L.; Blokland, M.; van der Wal, A.; Langenhoff, A. Biodegradation and adsorption of micropollutants by biological activated carbon from a drinking water production plant. J. Hazard. Mater. 2020, 388, 122028.
