Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
Tumor-associated macrophages (TAMs) play major roles in solid tumor development. They can have both anti-tumor and pro-tumor properties depending on their polarization.
- macrophages
- bladder cancer
- macrophage-targeting immunotherapy
1. Introduction
1.1. Tumor-Associated Macrophages
Macrophages are phagocytic immune cells found in most tissues, including healthy bladder [1], with diverse functionalities. They are essential in maintaining homeostasis through their sentinel functions and ability to adapt and respond to physiological changes or challenges from the outside. They maintain tissue integrity by eliminating damaged cells and matrices and play a role during development, regulating tissue remodeling. Moreover, they are important players in host defense and partake in the immune response.
In the mouse bladder, a large population of macrophages is present in the submucosa of the bladder and increases upon infection [2]. Their activation through pattern recognition receptors (PRRs) and intracellular receptors, such as the inflammasomes, triggers cytokine and chemokine production [3]. Macrophages have been shown to negatively affect the development of adaptive immunity to urinary tract infection [1,4] and although the functions of macrophages in bladder immunity, tissue integrity and homeostasis are not well understood, there is mounting evidence that resident macrophages can have a negative impact in bladder disease.
In cancer, macrophages are one of the major populations of infiltrating leukocytes in solid tumors [5]. Tissue-resident macrophages or monocyte-derived macrophages can infiltrate the tumor and are then called tumor-associated macrophages (TAMs). In the tumor, these cells can present two extreme phenotypes across a continuum of activation states, which are known as M1-like (pro-inflammatory) and M2-like (anti-inflammatory) TAMs [6]. M1-like TAMs have been reported to inhibit tumor development, progression, angiogenesis and promote adaptive immune responses through the secretion of pro-inflammatory cytokines [7]. M1-like TAMs can kill tumor cells through, for instance, nitric oxide (NO) production. They also have the capacity to present tumor antigen to Th1 CD4+ T cells and drive the activity of cytotoxic CD8+ T cells [8]. During tumor progression, tumor cells can subvert TAMs to prevent M1-like accumulation and favor M2-like pro-tumor TAMs. These macrophages stimulate tumor initiation, progression and survival. They promote tumor growth and angiogenesis by providing cell growth factors and angiogenic molecules [9]. M2-like TAMs also favor cancer metastasis, as they stimulate tumor cell motility, invasion, and extravasation. In addition, M2-like TAMs have the capacity to secrete anti-inflammatory cytokines or inhibitory molecules, such as programmed death-ligand 1 (PD-L1) [10]. As a result, the activity of cytotoxic CD8+ T cells is suppressed, preventing tumor cell elimination [11]. Likewise, CD4+ T cells are prompted to differentiate into regulatory T cells, known to contribute to immune response suppression [12].
To characterize macrophages, specific robust markers and markers to differentiate M1- from M2-like TAM are still subject of debate [13]. Macrophages have two origins. During embryonic development, the yolk sac gives rise to organ-resident macrophages which are locally self-maintained [14]. However, upon infection or tissue damage, macrophages are released from the bone marrow as immature monocytes that will circulate in the blood until they reach the targeted site. Two monocyte subsets can be described and present with different chemokine receptors and surface molecules: the inflammatory CD11 b+Ly6 Chi (CD14+CD16− in human) and the patrolling CD11 b+Ly6 Clow (CD14+CD16+ in human) monocytes [15]. Once monocytes enter the target tissue, they differentiate into macrophages expressing F4/80 and CD11 b in mice and CD14, CD68 and CD16 in humans [16,17]. Inducible nitric oxide synthase (iNOS) and arginase 1 (Arg1) can be used to describe M1- and M2-like TAM, respectively [18]. Other M2-like TAM markers were used, such as CD163, CD204, CD206, DC-SIGN, Galectin-9 or hypoxia-inducible factor-2α (HIF-2α). They were generally used alone in immunohistochemistry, making a comparison of possible different populations of M2-like macrophages impossible. We will further discuss these populations and their importance in the context of BCa. Within the same tumor, it has been shown that M1-like and M2-like TAMs can coexist. However, in advanced tumors, TAMs generally present an M2-like state that correlates with poor cancer prognosis [19].
A closely related subtype to macrophages which can also be recruited to the site of inflammation is the myeloid-derived suppressor cell (MDSC). MDSCs are known to be immature cells deriving from the myeloid lineage. These cells are a heterogeneous population, but in this review, we will focus on one subtype, the monocytic MDSCs (M-MDSCs), expressing CD11 b+Ly6 ChighLy6 G− in mice and CD33+CD14+HLA-DRlowCD15− in human [12]. It is still unclear whether these cells give rise to macrophages or represent a specific terminal population [11]. The initial defining feature of MDSCs is their activity to suppress the adaptive immune response, thus, potentially influencing the fate of certain diseases, such as cancer.
1.2. Bladder Cancer
Bladder cancer (BCa) represents the fourth most common cancer type in men [20], with about 430,000 new cases per year worldwide [21]. The most common forms of BCa are urothelial carcinomas, which are classified using the tumor–node–metastasis system (TNM system) that characterizes first the degree of invasion of the primary tumor (pathologic T (pT) stage from pTis to T4), then tumor spreading to nearby lymph nodes, and lastly, whether there is a development of distant metastases. These tumors are also graded according to the cellular anaplasia which predicts the low-risk (low grade) or the high-risk (high grade) of tumor growth, spreading or recurrence [22].
Among urothelial carcinomas, about 75% are a non-muscle-invasive bladder cancer (NMIBC, pTis-T1) where the tumor localizes to the urothelium or lamina propria but does not invade the muscle layers. The other 25% of urothelial carcinomas are a muscle-invasive bladder cancer (MIBC, pT2-T4) and metastatic disease [22]. Within 5 years, between 50% and 70% of NMIBC will recur and 10% to 30% will progress toward MIBC or metastatic disease despite treatments [21]. For MIBC, the 5-year survival is about 50% with 50% of patients developing metastases. Patients with metastatic disease have a median survival of 15 months [23]. These numbers highlight the urgent need to improve treatment management for BCa patients, but this first requires a detailed understanding of BCa pathogenesis.
2. Macrophages in Bladder Cancer
2.1. Association of Macrophages, Clinicopathological Features and Outcomes in Bladder Cancer
The tumor immune microenvironment of BCa remains poorly investigated compared to other solid tumors. Macrophages represent the most abundant infiltrating immune cells with a mean density of 14.55 cells/mm2 in the tumor core of MIBC [24]. Several studies on patient samples have reported that TAMs, and specifically M2-like TAMs, are present in both the stroma and the tumor core of BCa [24,25,26,27].
In NMIBC, TAM infiltration is less intense in the bladder wall compared to normal control bladder wall [28]. Studies analyzing NMIBC and MIBC cohorts have reported that TAM count, determined by the pan-macrophage marker CD68, positively correlates with high pathologic T stages and a high grade [29,30,31,32,33]. However, two studies have declared no correlation between TAM count, detected by CD204 or CD163 markers, and tumor stage or grade [34,35]. One explanation for these opposite results may be the use of unique and different markers to detect TAMs in human tissues emphasizing the lack of robust macrophage markers for pathologists [13].
The CD163+ M2-like phenotype is also associated with tumor stage and grade in patients with BCa [26,36,37,38]. Wang and colleagues have reported that the localization of M2-like macrophages in the tumor tissue is important to consider as their density in the stroma, but not in the tumor core, is positively associated with tumor stage [25]. The correlation between TAMs, especially M2-like TAMs, and grade and stage of BCa has been confirmed at the largest scale by RNA-seq on the TCGA MIBC cohort [38]. In support of this, it was observed in a genetically-engineered mouse model of BCa that TAM number, composed of M2-like TAMs, increases with tumor progression [39]. However, TAM count is not associated with any other clinicopathological feature such as gender, age, tumor volume or multifocality in BCa [26,32,34].
In peripheral blood, CD33+ and CD14+ myeloid cells increase in BCa patients compared to healthy donors [32,40,41]. In parallel to TAMs, the level of circulating myeloid cells is higher in BCa patients with pT2-T4 stages than with pTa-T1 stages [32] suggesting a communication between the systemic and tumor inflammation.
The increase of TAMs with malignant progression suggests a role of these cells in BCa aggressiveness and clinical outcome. The presence of TAMs has been correlated with several unfavorable clinical outcomes in different solid tumors, including BCa (Table 1). Additionally, TAMs are associated with tumor recurrence in NMIBC patients [42,43]. In both NMIBC and MIBC patients, a high number of TAMs is associated with a higher risk of tumor progression [31,44], as well as a worse progression-free survival (PFS) and overall survival (OS) [27,29,35,45]. Depending on the phenotype and localization, macrophages can be associated with opposite clinical outcomes in BCa. By using DC-SIGN and CD68 to characterize M2-like TAMs, it was shown that they may contribute to tumor progression and poor prognosis [46]. Study of CD204+ staining of macrophages showed that their presence in the tumor stroma, but not in the tumor core, is associated with poor OS [25]. Using a broader M2-like signature, TCGA database analyses revealed that M2-like TAMs are significantly associated with a decreased OS and disease-free survival (DFS) [38,47]. In the blood, patients with a high level of circulating myeloid cells have a poor OS [32]. Conversely, CD169+ M1-like macrophages in the tumor-draining lymph nodes [48], but not in the tumor [25], are positively correlated with a favorable prognosis in MIBC patients. However, single-cell RNA-seq from immune-infiltrating cells revealed the wide diversity of TAMs in MIBC patients [49]. In this study, six different subsets of monocytes/macrophages were found in MIBC tumor, and their gene signatures did not correlate with the classical M1-like versus M2-like signatures. Moreover, the authors also identified another subset of TAM that shares both TAM-like and MDSC-like gene signatures [49], supporting the theory of the MDSC-to-macrophage differentiation. In the future, determining the origin of TAM (from tissue-resident macrophages, monocytes-derived macrophages or MDSC differentiation) could improve knowledge on TAM diversity and complexity but this is still limited by the lack of specific markers for each subtype. Altogether, this shows how complex it is to classify these cells by using a limited number of markers, leading to possible false positive count [13].
Table 1. Macrophages and bladder cancer clinical outcomes.
| Cell Types and Markers | Bladder Cancer Cohorts | Types of Sample | Findings | References |
|---|---|---|---|---|
| CD68+ (TAM) | 40 pTa-pT1 23 ≥ pT2 |
FFPE tissue | High TAM count was associated with poor 5-year survival | Hanada et al. [29] |
| CD68+ (TAM) | 81 pTa-pT1 11 pT2 |
FFPE tissue | High number of TAMs was significantly associated with risk of progression | Bostrom et al. [31] |
| CD68+ (TAM) | 112 pTa 89 pT1 93 MIBC |
FFPE tissue | High CD68/CD3 ratio was associated with poor OS | Sjödahl et al. [45] |
| CD68+ (TAM) | 10 low grades 34 high grades |
FFPE tissue | High TAM count was associated with poor DFS but not OS | Chai et al. [50] |
| CD163+ (TAM) | 115 MIBC | FFPE tissue | High TAM count was associated with poor PFS and OS | Xu et al. [35] |
| CD163+ (TAM) | 94 high grade pT1 | FFPE tissue | TAMs were associated with tumor recurrence and progression | Yang et al. [43] |
| CD204+round cells (TAM) | 155 NMIBC | FFPE tissue | High number of TAMs correlated with a high risk of recurrence | Miyake et al. [42] |
| TAM | MIBC (no metastatic disease) | TCGA database | A signature with low T cells, low NK cells, high Treg and high TAMs is associated with poor DFS and OS | Fu et al. [24] |
| TAM | 406 MIBC | TCGA database | Patients in the high-risk group had a signature with low CD8+ T cells, CD4+ T cells and high abundance of M0 macrophages | Li et al. [44] |
| CD204+CD68+ (CD204+ macrophages) | 212 pTa-pT1 90 pT2-pT4 |
FFPE tissue | High number of CD204+ macrophages in tumor stroma was associated with poor OS | Wang et al. [25] |
| DC-SIGN+CD68+ (DC-SIGN+TAM) | 257 MIBC | FFPE tissue | DC-SIGN+ TAMs may contribute to progression and poor prognosis | Hu et al. [46] |
| M2-like TAM | 429 MIBC | TCGA database | High M2-like TAM signature was associated with poor OS and DFS | Xue et al. [38] |
| M2-like TAM | 402 MIBC | TCGA database | M2-like TAM signature was associated with significantly worse 5-year OS and DFS outcomes | Jiang et al. [47] |
| CD169+CD68+ (CD169+macrophages) | 44 MIBC | FFPE tissue | CD169+ macrophages in tumor-draining lymph nodes were positively correlated with a favorable prognosis | Asano et al. [48] |
| CD33+ (MDSC) | 70 NMIBC 27 MIBC |
FFPE tissue | The number of tumor-infiltrating CD33+ MDSCs was significantly inversely correlated with patient OS | Zhang et al. [27] |
| CD11b+CD33lowHLA-DR− (MDSC) | 71 pTa-pT1 42 pT2-pT4 |
PBMC | High numbers of circulating CD11b+ CD33lowHLA-DR− cells were correlated with poor OS | Yang et al. [32] |
CSS: cancer-specific survival; DFS: disease-free survival; FFPE: formalin-fixed paraffin-embedded; MDSC: myeloid-derived suppressor cell; MIBC: muscle-invasive bladder cancer; NK cells: natural killer cells; NMIBC: nonmuscle-invasive bladder cancer; OS: overall survival; PBMC: peripheral blood mononuclear cell; PFS: progression-free survival; TAM: tumor-associated macrophage; TCGA: The Cancer Genome Atlas; Treg: regulatory T cells.
In summary, despite possible caveats related to the limited number of markers used to characterize TAM, M2-like TAMs were associated with higher tumor grade and bad prognosis for patients. Understanding how BCa favors this accumulation becomes critical in finding new strategies to prevent this accumulation and to treat patients.
2.2. Bladder Cancer Recruits TAMs
Tumor cells are the first actors to influence macrophages recruitment, as they are known to produce several chemokines and cytokines. In several solid tumors, macrophages are recruited to the tumor site through a gradient of chemotactic molecules, such as CCL2 (also known as MCP-1), colony stimulating factor 1 (CSF-1, also known as M-CSF) or several CXCL chemokines. In BCa (Figure 1), tumor cells are implicated in macrophage recruitment via the secretion of CCL2 [51] and MIF/CXCL2 [27]. However, the tumor is heterogeneous and different tumor clones can be found in the same tumor mass and can affect macrophages differently. Cheah and colleagues indicated that CD14high bladder tumor cells developed more vascularized and more infiltrated tumors than did CD14low bladder tumor cells. This was explained by the fact that CD14high tumor cells have a higher production of IL6, IL8/CXCL5, CXCL1, CXCL2, M-CSF, VEGF-A, FGF-2 than CD14low tumor cells [52]. Tumor heterogeneity is also the result of the oxygen level in the tumor microenvironment where hypoxia is a common characteristic. In BCa, TAM infiltration is higher in tumors presenting a high expression of HIF-1α or HIF-2α [50,53] and they are particularly concentrated in the hypoxic/necrotic areas of the tumor core [54], suggesting that hypoxic cells synthetize molecules that recruit TAMs in these specific areas. These mechanisms of TAM recruitment in BCa are potential new targets as we will discuss later in this review.
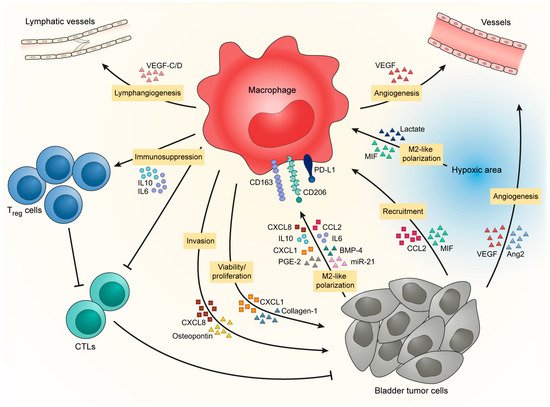
Figure 1. BCa favors M2-like polarization of TAM to promote tumor progression.
2.3. Bladder Cancer Favors M2-like Polarization of TAM to Promote Tumor Progression
In addition to TAM recruitment, bladder tumors also influence TAM phenotype by inducing the establishment of an M2-like phenotype that will enhance tumor development in return (Figure 1). Bladder tumor cells increase expression of CD206, CD163, PD-L1 and IL10 in macrophages [55,56] through the secretion of IL10 [57], chemokines [42,51], metabolic products [55,58], growth factors [37] and micro-RNA via exosome exchange [59]. Moreover, hypoxic areas, where TAMs are particularly concentrated in BCa [54], favor M2-like macrophages [60] through intracellular signaling and tumor-derived metabolites [61,62], which could contribute to the accumulation of pro-tumor macrophages in BCa.
In return, M2-like macrophages provide support for tumor progression. They contribute to an increase in bladder tumor cell proliferation and viability by the secretion of CXCL1 and collagen-I [63,64]. Macrophage count is also correlated with lymphatic metastasis underlying their role in the development of metastasis [29,35,65]. Macrophages promote lymphangiogenesis by the secretion of VEGF-C/D [51,66] and increase the ability of bladder tumor cells to form colonies and generate tumor spheres [56,67]. Macrophages further favor the development of metastasis by inducing tumor cell invasion through the production of CXCL8 and osteopontin [33,59,68,69]. This, together with the fact that in the tumor core TAMs are particularly concentrated at the proximity of basal-like layer/invasive front [37], indicates that macrophages could be involved in the transition from NMIBC to MIBC by supporting the invasion of tumor cells through the muscle layers. Moreover, because of the positive correlation between TAM counts and micro-vessel counts [42,63,70] and because of their localization in hypoxic/necrotic areas, TAMs are suspected to promote tumor angiogenesis. Macrophages provoke angiogenesis directly by producing VEGF [71] or indirectly by stimulating tumor cells to produce pro-angiogenic factors [67,68,72]. Finally, macrophages help to promote tumor progression by their capacity to inhibit anti-tumor immunity. Two studies have shown from fresh human tissues that CCR8+ TAMs and hyaluronidase 2-expressing TAMs increase the level of inflammatory factors in bladder tumor tissues, such as IL6, CXCL8 and CCL2 [71,72]. In parallel, CCR8− TAMs secrete CCL1 that will activate CCR8 on nearby TAMs increasing tumor inflammation [71]. In this study, Eruslanov and coworkers stated that CCR8+ TAMs are able to induce FoxP3 in lymphocytes, thus favoring regulatory T cells (Tregs) in the tumor microenvironment of BCa. The accumulation of Tregs in the bladder tissue is promoted by DC-SIGN+ TAMs [46]. The accumulation of suppressor lymphocytes is accompanied by a reduction of cytotoxic lymphocytes coordinated by DC-SIGN+ TAMs and PD-L1-expressing myeloid cells [46,55,57]. Furthermore, the expression of Galectin-9 by TAMs seems to promote exhaustion in T cells as they are associated with a decrease of IFNγ, GrzB, PRF1 and an increase of PD-1 and Tim3 in CD45+ cells [73]. Suppression of myeloid cells leads to an increased infiltration of CD8+ T cells in the tumor [74]. It seems that several specific subsets of M2-like macrophages are present in the environment of BCa, each having a specific role in tumor development. This concept shows the difficulty in finding common markers that can target all M2-like macrophages without depleting M1-like macrophages. M1-like macrophages can be important for the anti-tumor response during BCa therapy.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13184712
This entry is offline, you can click here to edit this entry!
