This study reports the application of antimicrobial peptides (AMPs) in food preservation because of their ability to inactivate spores and spore-forming bacteria. The review focused more on nisin, which is one of the sufficiently characterised AMPs for use in the food industry. The mechanisms of spore-forming bacteria inactivation, the effectiveness of using nisin AMP alone and the synergistic effect of combining AMPs with other non-thermal emerging technologies were explored.
- bacteriocin
- spores
- food processing
- food safety
- antimicrobial peptides
1. Introduction
Food preservation is one of the major challenges in the food industry. This is because resistant bacterial spores are perfect vehicles for spoiling food and infecting humans. Hence, the fight against foodborne illness and food spoilage due to spore-forming bacteria has become a major public health problem. As a result, the inactivation of bacterial endospores is a critical step in the food processing industry to ensure consumer safety and stable shelf-life of foods [1]. Micro-organisms produce several chemical agents as primary or secondary metabolites during their growth. These agents serve various functions related to the growth, metabolism of complex nutrients and aid in competition. One of such chemical agents is the antimicrobial peptides (AMPs), also known as bioactive peptides, bacteriocins or antimicrobial activity peptides [2][3][4]. AMPs are assorted and abundant clusters of biomolecules and natural proteins domicile in animals, plants and bacteria responsible for the defence of the host from pathogenic organisms [5][6]. As host defence peptides, AMPs can be classified as being cationic (positively charged) and amphiphilic (hydrophilic and hydrophobic) α-helical peptide molecules [7]. The methodology used in synthesising AMPs includes chemical, enzymatic and recombinant techniques.
AMPs offer alternatives to the chemical preservatives of foods to improve shelf-life. Presently, nisin is the only AMP extensively employed as a bio-preservative in food [5]. The utilisation of bio-preservatives offers an advantage over chemical preservatives such as nitrites and sulphur dioxide, which could adversely impact the quality and nutrition level of foods and human health. Therefore, the major benefits of the AMPs in the preservation of foods include no alteration of quality and it is not harmful [8]. AMPs are widely studied due to their natural antimicrobial properties and a broad spectrum of activity against bacteria, fungi, and viruses. In this regard, AMPs produced by bacteria, insects, amphibians and mammals, and those synthesised chemically are potential candidates for the design and development of new antimicrobial agents.
A wide range of bacteria synthesizes AMPs such as bacteriocins, which are potential alternatives to traditional antibiotics. These peptides have high potency and low toxicity and can be produced in situ by probiotics or bioengineered. However, the most predominant producers are the lactic acid bacteria (LAB) that easily break down lactose and other sugars to produce lactic acid, diacyl, hydrogen peroxide and other metabolites [9]. The production of bioactive peptides is an adaptation mechanism, aiding competition. These peptides exert a bactericidal or bacteriostatic effect against closely related strains of the producer organism and other bacteria genera [10]. Although AMPs inhibit the growth of bacteria, they are not antibiotics. The difference between bacteriocins and antibiotics is that whilst antibiotics are secondary metabolites and enzymatically synthesized, bacteriocins are primary metabolites and are ribosomally synthesized [11]. Nisin is an example of AMPs produced by Lactococcus lactis with antimicrobial activity against several Gram-positive bacteria [12]. Nisin has shown low toxicity as well as antibacterial activity, which proves it's used as a food preservative [13].
In food processing and preservation, there is growing interest by consumers in minimally processed foods that have not been subjected to rigorous thermal treatment, free from chemical preservatives but still microbiologically safe. Food production that fits these criteria is a challenge in the food industry as thermal and chemical treatments are widely employed for food safety and shelf-life elongation. Thus, novel approaches have to be used to achieve these and control potential microbial agents such as the spore formers Bacillus sp. and Clostridium sp. Traditionally, Bacillus sp. and Clostridium spores are eliminated from foods using extreme treatments, including high temperature and chemicals. Although these treatments effectively eliminate bacteria spores, they negatively affect the nutritional and organoleptic quality of the foods [14][15]. The use of antimicrobial peptides can be an approach for controlling these food spoilage/poisoning organisms in minimally processed foods. AMPs are generally classified into three, based on the producing strains, chemical composition, molecular weight, and mechanism of action [16]. Class I AMPs contains the uncommon amino acid lanthionine and are enzymatically modified during biosynthesis, Class II AMPs are small and unmodified with a size of about 10 kDa, while Class III are also unmodified but with a size larger than 10 kDa [17]. Currently, there are several characterized and purified bacteriocins. Some examples include; subtilin (Class I), thuricin (i.e., sactibiotic subclass of bacteriocin, class IId), cerein (class IIb sec-independent bacteriocin), and plantaricin (class IIa bacteriocin) processed for use as food additives/preservatives. However, the most common AMP is nisin (class I bacteriocin) which is produced by L. lactis and is generally regarded as safe (GRAS) for use in food products [18][19]. Figure 1 shows the molecular structure of nisin a typical AMP. Nisin has demonstrated a broad spectrum of antibacterial activity including activity against many pathogenic bacteria that are responsible for food spoilage. Hence, the safety and efficacy of nisin as a food preservative have resulted in widespread usage. Nisin is a known member of the lantibiotics AMPs, since it contains the uncommon amino acid lanthionine, with considerable potential in food preservation. Nisin has been satisfactorily characterized to be used for this purpose. For this reason, nisin has been approved by the United States Food and Drug Administration US-FDA [15].
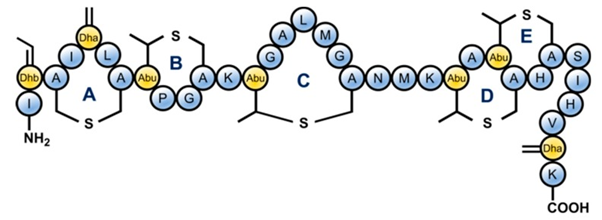
Figure 1. Structure of Nisin which is a lantibiotic AMP due to the presence of uncommon lanthionine rings in the structure
and also the unsaturated amino acids introduced by posttranslational modifications: Dhb = dehydrobutyrine, Dha = dehydroalanine and Abu = aminobutyric acid (Bolt, H.L.; Kleijn, L.H.; Martin, N.I.; Cobb, S.L.; Synthesis of Antibacterial Nisin–Peptoid Hybrids Using Click Methodology. Molecules 2018, 23(7), 1566, https://doi.org/10.3390/molecules23071566.)
AMPs in association with other treatments have been shown to be more effective in the control of microorganisms in foods. Consequently, the combined effect of high-pressure CO2 and nisin to inactivate Bacillus subtilis spores has been investigated and reported by Rao et al. [20]. It was discovered that the damaged spores coat and cortex by diffusing high-pressure CO2 aided the penetration of nisin into the spores’ inner membrane, resulting in higher inactivation. This is in agreement with the result of Li and co-workers [21], where high-pressure CO2 was combined with 0.02% nisin at 10 MPa and 32 ℃ for 15 min produced a superior inactivation of E. coli and Staphylococcus aureus than the application of high-pressure CO2 alone.
AMPs have the advantage of having a minimal impact on the organoleptic quality of foods without the degradation of the nutritional value which may occur with the use of some chemical preservatives [22]. Furthermore, they are easily digested by the human gastrointestinal tract; thus they do not get into the systemic circulation, reducing the possibility of having suboptimal levels in the body which can lead to the development of antimicrobial drug resistance [23][24]. Within food matrices, AMPs remain stable in wide pH and temperature ranges, retaining their antimicrobial activity after processing treatments that the foods receive [25]. This review focuses on the progress that has been made on the use of AMPs such as nisin to inactivate spore-forming bacteria and spores. It discusses the different types of AMPs, purification methods, mode of action and mechanisms of inactivating spore-forming bacteria and spores in order to preserve food and enhance shelf-life without impacting on quality and nutritional attributes.
2. Inactivation of Bacterial Spores by AMPs
Bacteria spores are abundant in the environment and can contaminate foods during different production processes [26]. Control of spores and spore-forming microbes in food is important because of their resilient nature, as their survivability in processed foods and subsequent germination under favourable conditions would shorten shelf-life, cause spoilage and food poisoning. In addition to food spoilage, foodborne illnesses resulting from toxin production can occur [27], especially in improperly canned foods. For instance, Bacillus and Clostridium species are known for producing emetic toxins, exo and neurotoxins, which are responsible for specific symptoms associated with the consumption of contaminated foods. The formation of bacterial toxins by Clostridium and Bacillus sp. is usually achieved by vegetative cells after germination of the spores and during growth [27]. Thus, for ensuring food safety, the spores of these bacteria genera need not be eliminated in totality but rather suppressed from germinating in foods.
Generally, the control of spores and spore-forming bacteria is achieved traditionally by prolonged heating targeted at eliminating the vegetative forms of the organism to prevent spore formation. However, this method of preservation can negatively affect food quality (both nutritionally and food acceptance) and cannot be applied to some food products particularly proteinous foods which are heat sensitive. The alternative approach, therefore, is to control, suppress and prevent the microbial spores in foods from emerging. This can be achieved with the aid of antimicrobial peptides, which has demonstrated potential inhibiting spores’ germination or outgrowth. This is usually achieved by the direct inclusion of AMP into the food or the fermentation of foods using bacteriocinogenic bacteria which utilises food substrate and synthesize the antimicrobial peptides directly into the food product. The functions of the AMPs depend not only on their specific amino acid components and three-dimensional structure but also on their interfacial activity. The interfacial properties and the physicochemical interactions are important factors that control the biological activities of these AMPs with the membrane-destabilizing and membrane-permeabilizing abilities [7]. Hence, the amino acid composition, amphipathicity, helicity, cationicity and size enhance their insertion into lipid membranes, resulting in the inactivation of the target spore-forming microbes [28]. It is evident that AMPs possess some specific structures and features that allow them to interact with, bind to, and disrupt cell membranes. In 2013, Vicente et al. studied the interactions between a membrane mimetic and the cationic AMP Ctx(Ile 21)-Ha, containing the paramagnetic amino acid 2,2,6,6-tetramethylpiperidine-1-oxyl-4-amino-4-carboxylic acid (TOAC) incorporated at residue positions n = 0, 2, and 13. It was observed with the aid of fluorescence experiments that all peptides were able to interact with lysophosphocholine micelles.
In a study by Oman and van der Donk [29], haloduracin, a bacteriocin produced by Bacillus halodurans C-125 inhibited the germination and spore outgrowth of Bacillus anthracis. In another study by Martínez-Cuesta et al. [30], a lacticin 3147-producing L. lactis demonstrated the ability to control the outgrowth of Clostridium spores in semi-hard cheese. Other strategies in using antimicrobial peptides in food processing for the control of spores involve inoculation of foods with bacteriocinogenic lactic acid bacteria (LAB) for in-situ production of bacteriocins. This has been applied in the control of Clostridium tyrobutyricum [31], using AMPs-producing Lactobacillus gasseri K7 in cheese. These potent abilities of bacteriocinogenic LAB to inhibit and prevent the outgrowth of bacteria spores show that LABs, when utilized as a starter culture in fermentations, perform a dual role which includes microbial fermentation and food preservation. This is bearing in mind that many bacteriocinogenic LAB are originally isolated as part of food flora and are already responsible for wild type fermentations in these foods. Thus, when used within related food systems, they can result in food fermentations without undesirable metabolites. This approach has been employed in several studies to control mainly vegetative cells and spores as demonstrated in the study by Garde et al. [32]. They isolated L. lactis subsp. lactis INIA 415, a bacteriocinogenic LAB that produces nisin Z and Lacticin 481 from Manchego cheese and utilized this in the fermentation of Hispanico cheese to develop volatile compounds and preservation [33]. In a subsequent study by Garde et al. [34], the outgrowth/germination of Clostridium heijerinckii spores was inhibited by AMP-producing lactic acid bacteria culture in ovine milk cheese. Compared to naturally fermented uncontaminated cheese samples, contamination of the cheese with Clostridium heijerinckii while fermenting with a bacteriocinogenic LAB resulted in a late blowing effect; thus, highlighting suppressed sporulation of the vegetative cells [34]. Similar sporicidal and sporostatic effect of AMPs such as nisin has been reported in earlier studies [35]. Table 1 displays some AMPs that have proven effective against bacterial spores. Their antimicrobial mechanisms are different from those of traditional antibiotics. The mechanism of action is based on the ability of AMPs to alter membrane permeability and promote decomposition of the spore-forming microbe cell membrane. Furthermore, they can act on different targets in the cells such as DNA, RNA, regulatory enzymes, and other proteins [36].
| AMPs | Spore Former | References |
|---|---|---|
| Nisin | C. perfringens, C. sporogenes, C. botulinum, C. difficile, C. beijerinckii | [37][38][39] |
| Enterocin | A. acidoterrestris, B. cereus, B. licheniformis, G. stearothermophilus | [40][41] |
| Bificin | A. acidoterrestris | [42] |
| Lacticin | C. tyrobutyricum | [30] |
| Plantaricin | C. sporogenes | [43] |
| Thurincin | B. cereus | [44] |
To date, only nisin and pediocin PA-1 have been satisfactorily characterized to be used in the food industry as bio-preservatives [45]. In other words, there is a need to sufficiently characterise other AMPs for use in food preservation.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26185552
References
- Reineke, K.; Mathys, A. Endospore Inactivation by Emerging Technologies: A Review of Target Structures and Inactivation Mechanisms. Annu. Rev. Food Sci. Technol. 2020, 11, 255–274.
- Mojgani, N.; Amirnia, C. Kinetics of Growth and Bacteriocin Production in L. casei RN 78 Isolated from a Dairy Sample in IR Iran. Int. J. Dairy Sci. 2017, 2, 1–12.
- Preciado, G.M.; Michel, M.M.; Villarreal-Morales, S.L.; Flores-Gallgos, A.C.; Aguirre-Joyal, J.; Morlett-Chavez, J.; Aguilar, C.N.; Rodriguez-Herrera, R. Bacteriocins and Its Use for Multidrug-Resistant Bacteria Control. In Antibiotic Resistance; Academic Press: Cambridge, MA, USA, 2016; Chapter 16.
- Chandrakasan, G.; Rodríguez-Hernández, A.I.; Del Rocío López-Cuellar, M.; Palma-Rodrı’guez, H.-M.; Chavarrı’a-Herna’ndez, N. Bacteriocin encapsulation for food and pharmaceutical applications: Advances in the past 20 years. Biotechnol. Lett. 2019, 41, 453–469.
- Rai, M.; Pandit, R.; Gaikwad, S.; Ko¨vics, G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J. Food Sci. Technol. 2016, 53, 3381–3394.
- Silveira, F.R.; Roque-Borda, A.C.; Vicente, F.E. Antimicrobial peptides as a feed additive alternative to animal production; food safety and public health implications: An overview. Anim. Nutr. 2021.
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, H.D.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931.
- Wang, S.; Zeng, X.; Yang, Q.; Qiao, S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci. 2016, 17, 603.
- Alakomi, H.L.; Skytta, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005.
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241.
- Jamaluddin, N.; Stuckey, D.C.; Ariff, A.B.; Wong, F.W.F. Novel approaches to purifying bacteriocin: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2453–2465.
- Kramer, N.E.; Smid, E.J.; Kok, J.; De Kruijff, B.; Kuipers, O.P.; Breukink, E. Resistance of Gram-positive bacteria to nisin is not determined by lipid II levels. FEMS Microbiol. Lett. 2004, 239, 157–161.
- Martin, N.I.; Breukink, E. The expanding role of lipid II as a target for lantibiotics. Future Microbiol. 2007, 2, 513–525.
- Marriott, B.M. Heat as a Factor in the Perception of Taste, Smell, and Oral Sensation. In Nutritional Needs in Hot Environments: Applications for Military Personnel in Field Operations; National Academies Press: Washington, DC, USA, 1993.
- Hart, A.; Anumudu, C.; Onyeaka, H.; Miri, T. Application of supercritical fluid carbon dioxide in improving food shelf-life and safety by inactivating spores: A review. J. Food Sci. Technol. 2021, 1–12.
- Kaskoniene, V.; Stankevicius, M.; Bimbiraite-Surviliene, K.; Naujokaityte, G.; Serniene, L.; Mulkyte, K.; Malakauskas, M.; Maruska, A. Current state of purification; isolation and analysis of bacteriocins produced by lactic acid bacteria. Appl. Microbiol. Biotechnol. 2017, 101, 1323–1335.
- Alvarez-Sieiro, P.; Montalban-Lopez, M.; Mu, D.D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl Microbiol. Biotechnol. 2016, 100, 2939–2951.
- Ogunbanwo, S.T.; Sanni, A.I.; Onilude, A.A. Characterization of bacteriocin produced by Lactobacillus plantarum F1 and Lactobacillus brevis OG1. Afr. J. Biotechnol. 2003, 2, 219–227.
- Ghrairi, T.; Chaftar, N.; Hani, K. Bacteriocins: Recent Advances and Opportunities. Prog. Food Preserv. 2012, 485–511.
- Rao, L.; Wang, Y.; Chen, F.; Liao, X. The Synergistic Effect of High Pressure CO2 and Nisin on Inactivation of Bacillus subtilis Spores in Aqueous Solutions. Front. Microbiol. 2016, 7, 1507.
- Li, H.; Xu, Z.; Zhao, F.; Wang, Y.; Liao, X. Synergetic effects of high- pressure carbon dioxide and nisin on the inactivation of Escherichia coli and Staphylococcus aureus. Innov. Food Sci. Emerg Technol. 2016, 33, 180–186.
- Prudêncio, C.V.; Dos Santos, M.T.; Vanetti, M.C.D. Strategies for the use of bacteriocins in Gram-negative bacteria: Relevance in food microbiology. J. Food Sci. Technol. 2015, 52, 5408–5417.
- Mills, S.; Serrano, L.M.; Griffin, C.; O’Connor, P.M.; Schaad, G.; Bruining, C.; Hill, C.; Ross, R.P.; Meijer, W.C. Inhibitory activity of Lactobacillus plantarum LMG P-26358 against Listeria innocua when used as an adjunct starter in the manufacture of cheese. Microb. Cell Fact. 2011, 10, 11.
- Gough, R.; O’Connor, M.P.; Rea, C.M.; Gomez-Sala, B.; Miao, S.; Hill, C.; Brodkorb, A. Simulated gastrointestinal digestion of nisin and interaction between nisin and bile. LWT 2017, 86, 530–537.
- Belgacem, Z.B.; Rehaiem, A.; Bernárdez, P.F.; Manai, M.; Castro, L.P. Interactive effects of pH and temperature on the bacteriocin stability by response surface analysis. Microbiology 2012, 81, 195–200.
- Julien, M.C.; Dion, P.; Lafreniere, C.; Antoun, H.; Drouin, P. Sources of clostridia in raw milk on farms. Appl. Environ. Microbiol. 2008, 74, 6348–6357.
- Adams, M.R.; Moss, M.O. Food Microbiology; RSC Publishing: Cambridge, UK, 2008.
- Vicente, E.F.; Basso, L.G.M.; Cespedes, G.F.; Lorenzon, E.N.; Castro, M.S.; Mendes-Giannini, M.J.S.; Cilli, E.M. Dynamics and conformational studies of TOAC spin labeled analogues of Ctx(Ile21)-Ha peptide from Hypsiboas albopunctatus. PLoS ONE 2013, 8, e60818.
- Oman, T.J.; Van der Donk, W.A. Insights into the mode of action of the two-peptide lantibiotic haloduracin. ACS Chem. Biol. 2009, 4, 865–874.
- Martínez-Cuesta, M.C.; Bengoechea, J.; Bustos, I.; Rodríguez, B.; Requena, T.; Pelaez, C. Control of late blowing in cheese by adding lacticin 3147-producing Lactococcus lactis IFPL 3593 to the starter. Int. Dairy J. 2010, 20, 18–24.
- Bogovic Matijasic, B.; Koman Rajsp, M.; Perko, B.; Rogelj, I. Inhibition of Clostridium tyrobutyricum in cheese by Lactobacillus gasseri. Int. Dairy J. 2007, 17, 157–166.
- Garde, S.; Rodríguez, E.; Gaya, P.; Medina, M.; Nuñez, M. PCR detection of the structural genes of nisin Z and lacticin 481 in Lactococcus lactis subsp. lactis INIA 415; a strain isolated from raw milk Manchego cheese. Biotechnol. Lett. 2001, 23, 85–89.
- Garde, S.; Carbonell, M.; Fernández-García, E.; Medina, M.; Nuñez, M. Volatile compounds in Hispánico cheese manufactured using a mesophilic starter; a thermophilic starter and bacteriocin-producing Lactococcus lactis subsp. lactis INIA 415. J. Agric. Food Chem. 2002, 50, 6752–6757.
- Garde, S.; Avila, M.; Arias, R.; Gaya, P.; Nunez, M. Outgrowth inhibition of Clostridium beijerinckii spores by a bacteriocin-producing lactic culture in ovine milk cheese. Int. J. Food Microbiol. 2011, 150, 59–65.
- De Vuyst, L.; Vandamme, E.J. Nisin; A Lantibiotic Produced by Lactococcus Lactis Subsp. Lactis: Properties; Biosynthesis; Fermentation and Applications. In Bacteriocins of Lactic Acid Bacteria; De Vuyst, L., Vandamme, E.J., Eds.; Blackie Academic & Professional: London, UK, 1994; pp. 151–221.
- Maria-Neto, S.; De Almeida, K.C.; Macedo, M.L.R.; Franco, O.L. Understanding bacterial resistance to antimicrobial peptides: From the surface to deep inside. Biochim. Biophys. Acta 2015, 1848, 3078–3088.
- Hofstetter, S.; Gebhardt, D.; Ho, L.; Ganzle, M.; McMullen, M. Effect of nisin and reutericyclin on resistanceof endospores of Clostridium spp. to heat and high pressure. Food Microbiol. 2013, 34, 46–51.
- Nerandzic, M.M.; Donskey, C.J. Activate to Eradicate: Inhibition of Clostridium difficile Spore Outgrowth by the Synergistic Effects of Osmotic Activation and Nisin. PLoS ONE 2013, 8, e54740.
- Aouadhi, C.; Mejri, S.; Maaroufi, A. Inhibitory effects of nisin and potassium sorbate alone or in combination on vegetative cells growth and spore germination of Bacillus sporothermodurans in milk. Food Microbiol. 2015, 46, 40–45.
- Abriouel, H.; Maqueda, M.; Gálvez, A.; Martínez-Bueno, M.; Valdivia, E. Inhibition of Bacterial Growth; Enterotoxin Production; and Spore Outgrowth in Strains of Bacillus cereus by Bacteriocin AS-48. Appl. Environ. Microbiol. 2002, 68, 1473–1477.
- Lucas, R.; Grande, M.J.; Abriouel, H.; Maqueda, M.; Omar, N.B.; Valdivia, E.; Gálvez, A. Application of the broad-spectrum bacteriocin enterocin AS-48 to inhibit Bacillus coagulans in canned fruit and vegetable foods. Food. Chem Toxicol. 2006, 44, 1774–1781.
- Pei, J.; Yue, T.; Jin, W. Application of bacteriocin RC20975 in apple juice. Food Sci. Technol. Int. 2016, 23, 166–173.
- González, L.; Zárate, V. Inhibitory activity of Lactobacillus plantarum TF711 against Clostridium sporogenes when used as adjunct culture in cheese manufacture. J. Dairy Res. 2015, 82, 236–241.
- Wang, G.; Manns, D.C.; Guron, G.K.; Churey, J.J.; Worobo, R.W. Large-scale purification; characterization; and spore outgrowth inhibitory effect of Thurincin H, a bacteriocin produced by Bacillus thuringiensis SF361. Probiotics Antimicrob. Proteins 2014, 6, 105–113.
- Zhou, H.; Fang, J.; Tian, Y.; Lu, Y.X. Mechanisms of nisin resistance in Gram-positive bacteria. Ann. Microbiol. 2014, 64, 413–420.
