COVID-19 pneumonia represents a challenging health emergency, due to the disproportion between the high transmissibility, morbidity, and mortality of the virus and healthcare systems possibilities. Literature has mainly focused on COVID-19 pneumonia clinical-radiological diagnosis and therapy, and on the most common differential diagnoses, while few papers investigated rare COVID-19 pneumonia differential diagnoses or the overlapping of COVID-19 pneumonia on pre-existing lung pathologies. This article presents the main radiological characteristics of COVID-19 pneumonia and Idiopathic Interstitial Pneumonias (IIPs) to identify key radiological features for a differential diagnosis among IIPs, and between IIPs and COVID-19 pneumonia. COVID-19 pneumonia differential diagnosis with IIPs is challenging, since these entities may share common radiological findings as ground glass opacities, crazy paving patterns, and consolidations. Multidisciplinary discussion is crucial to reach a final and correct diagnosis. Radiologists have a pivotal role in identifying COVID-19 pneumonia patterns, reporting possible overlapping with long-lasting lung diseases, and suggesting potential differential diagnoses. An optimal evaluation of HRTC may help in containing the disease, in promoting better treatment for patients, and in providing an efficient allocation of human and economic resources.
- COVID-19
- idiopathic interstitial pneumonia
- HRCT
- differential diagnosis
- usual interstitial pneumonia
- non-specific interstitial pneumonia
- organizing pneumonia
- acute respiratory distress syndrome
1. Introduction
Literature on COVID-19 pneumonia clinical-radiological diagnosis and therapy is extremely fertile and has mainly focused on the most common differential diagnoses [1][2][3], while few papers investigated rarer differential diagnoses and of COVID-19 pneumonia overlapping on long-lasting lung pathologies.
Idiopathic Interstitial Pneumonias (IIPs) represent a vast category of lung diseases with kaleidoscopic radiological, clinical, and histopathological features as defined by the 2013 Idiopathic Interstitial Pneumonia Classification of the American Thoracic Society/European Respiratory Society [4].
The radiologist has a pivotal role in identifying COVID-19 pneumonia radiological features, reporting its possible overlapping with long-lasting lung diseases, and suggesting potential differential diagnoses. An optimal evaluation of HRTC may promote better treatment for patients and provide an efficient allocation of human and economic resources.
Our main goals are to provide the main radiological characteristics of COVID-19 pneumonia and Idiopathic Interstitial Pneumonias and to identify key radiological features for a differential diagnosis among IIPs and between IIPs and COVID-19 pneumonia.
2. COVID-19 Pneumonia
| Stage | Phase | Timing (Days) | Predominant Radiological Findings | Additional Findings | Spatial Distribution of Radiological Findings |
|---|---|---|---|---|---|
| 1 | Early | 0–4 | ground glass opacities |
|
|
| 2 | Progressive | 5–8 | crazy paving pattern, ground glass opacities and small consolidations | ||
| 3 | Peak | 9–13 | consolidative foci | ||
| 4 | Absorption | ≥14 | ground-glass opacities and linear consolidation |
-
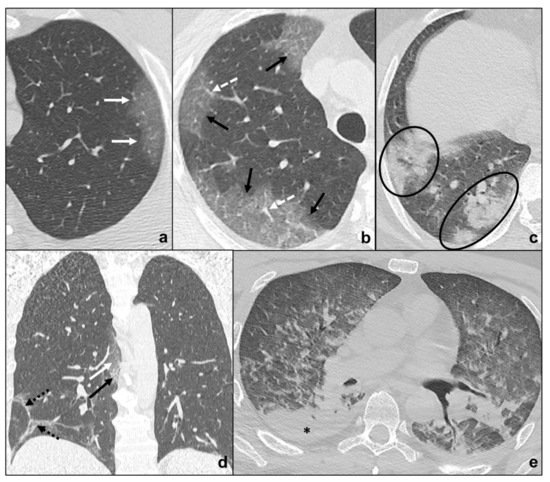
Early phase/Stage 1 refers to days 0–4, and ground glass opacities represent the main radiological findings (Figure 1a);
-
Progressive phase/Stage 2 refers to days 5–8, and the hallmark is represented by crazy paving pattern (Figure 1b) coexisting with extensive ground glass opacities and initial consolidative foci;
-
Peak phase/Stage 3 is typical of days 9 to 13, and CT shows consolidations (Figure 1c), sometimes surrounded by a ground glass halo (halo sign);
-
Absorption phase/Stage 4 starts around day 14, and ground-glass areas together with linear consolidations are appreciable (Figure 1d).
3. Idiopathic Interstitial Pneumonias
| Idiopathic Interstitial Pneumonias | Typical CT Features | Pattern of Distribution | ||
|---|---|---|---|---|
| Main Idiopathic Interstitial Pneumonias | Chronic Fibrosing Interstitial Pneumonias | Idiopathic Pulmonary Fibrosis | macrocystic honeycombing, architectural distortion, traction bronchiectases, irregular reticular opacities, decreased lung volumes, non-predominant ground glass opacities | apicobasal gradient, heterogeneous involvement, subpleural location |
| Non Specific Interstitial Pneumonia | no apicobasal gradient, basal predominance, homogeneous involvement, symmetric, peripheral, subpleural sparing | ground glass, irregular reticular opacities, traction bronchiectases | ||
| Smoking-Related Interstitial Pneumonias | Respiratory Bronchiolitis-Associated Interstitial Lung Disease | centrilobular nodules, bronchial wall thickening, ground glass opacities, centrilobular emphysema | diffuse or upper lobes predominance, centrilobular | |
| Desquamative Interstitial Pneumonia | ground glass, linear or reticular opacities, rare perivascular cysts | apicobasal gradient, peripheral | ||
| Acute/Subacute Interstitial Pneumonias | Cryptogenic Organizing Pneumonia | ground glass, consolidations, rare centrilobular nodules and reticular opacities | apicobasal gradient, unilateral or bilateral, patchy distribution, peribronchial or peripheral, migration tendency, possible subpleural sparing | |
| Acute Interstitial Pneumonia | exudative: patchy ground glass, consolidation; organizing: architectural distortions, traction bronchiectases, honeycombing | bilateral, symmetric, dependent lung segments, basal | ||
| Rare Idiopathic Interstitial Pneumonias | Lymphoid Interstitial Pneumonia | perivascular thin-walled cysts, ground glass, centrilobular nodules, septal thickening | bilateral, basal, or diffuse | |
| Idiopathic Pleuroparenchymal Fibroelastosis | fibrotic changes, pleural thickening, consolidations, architectural distortions, traction bronchiectasis, hilar elevation, progressive upper lobe volume loss | bilateral, upper field predominance, subpleural | ||
3.1. Major Idiopathic Interstitial Pneumonias
3.1.1. Chronic Fibrosing IIPs
Idiopathic Pulmonary Fibrosis
Non-Specific Interstitial Pneumonia
3.1.2. Smoking-Related IIPs
Respiratory Bronchiolitis-Associated Interstitial Lung Disease
Desquamative Interstitial Pneumonia
3.1.3. Acute/Subacute IIPs
Cryptogenic Organizing Pneumonia
Acute Interstitial Pneumonia
3.2. Rare Idiopathic Interstitial Pneumonias
3.2.1. Lymphoid Interstitial Pneumonia
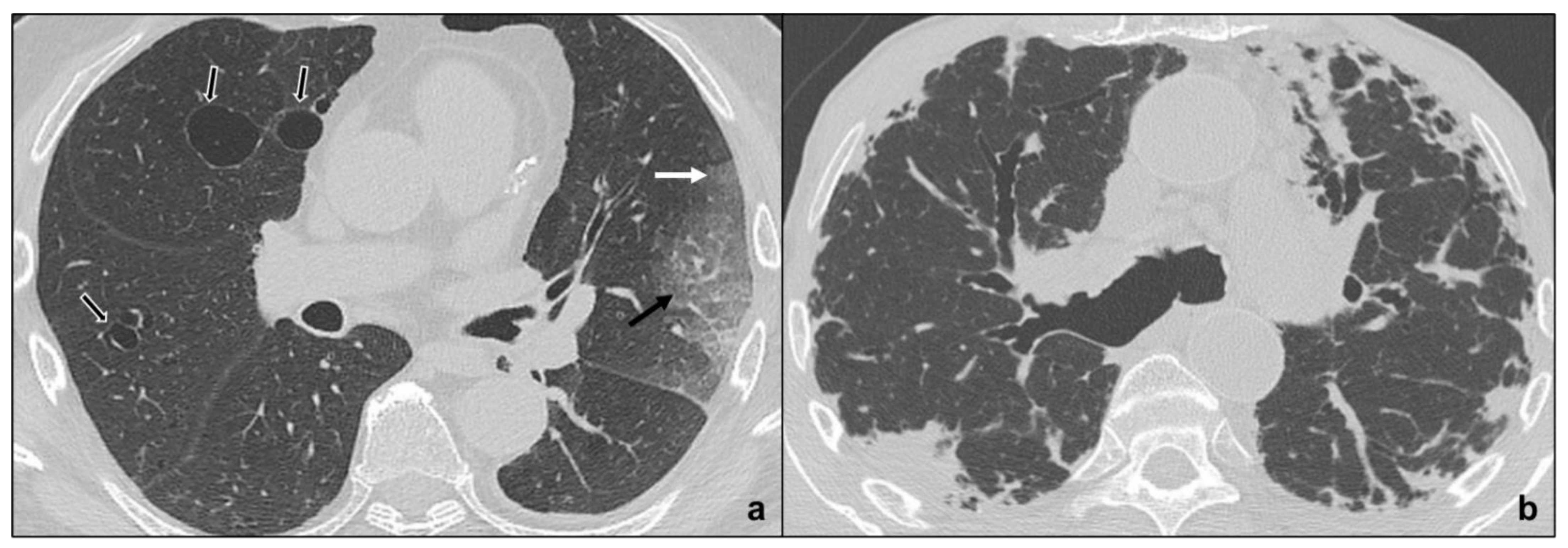
3.2.2. Idiopathic Pleuroparenchymal Fibroelastosis
3.3. Unclassifiable Idiopathic Interstitial Pneumonias
4. Differential Diagnosis between COVID-19 Pneumonia and Idiopathic Interstitial Pneumonias
| Idiopathic Interstitial Pneumonias | Ground Glass | Crazy Paving | Consolidations | ||
|---|---|---|---|---|---|
| Main Idiopathic Interstitial Pneumonias | Chronic Fibrosing Interstitial Pneumonias | Idiopathic Pulmonary Fibrosis | C (non predominant pattern, exacerbation) | R | R |
| Non-Specific Interstitial Pneumonia | C | OD/A | R | ||
| Smoking-Related Interstitial Pneumonias | Respiratory Bronchiolitis-Associated Interstitial Lung Disease | C | R | OD/A | |
| Desquamative Interstitial Pneumonia | C | OD/A | OD/A | ||
| Acute/Subacute Interstitial Pneumonias | Cryptogenic Organizing Pneumonia | C (initial exacerbation) | OD/A | C (late) | |
| Acute Interstitial Pneumonia | C (early) | C | C (early) | ||
| Rare Idiopathic Interstitial Pneumonias | Lymphoid Interstitial Pneumonia | C | C/R | R | |
| Idiopathic Pleuroparenchymal Fibroelastosis | OD/A | OD/A | C | ||
-
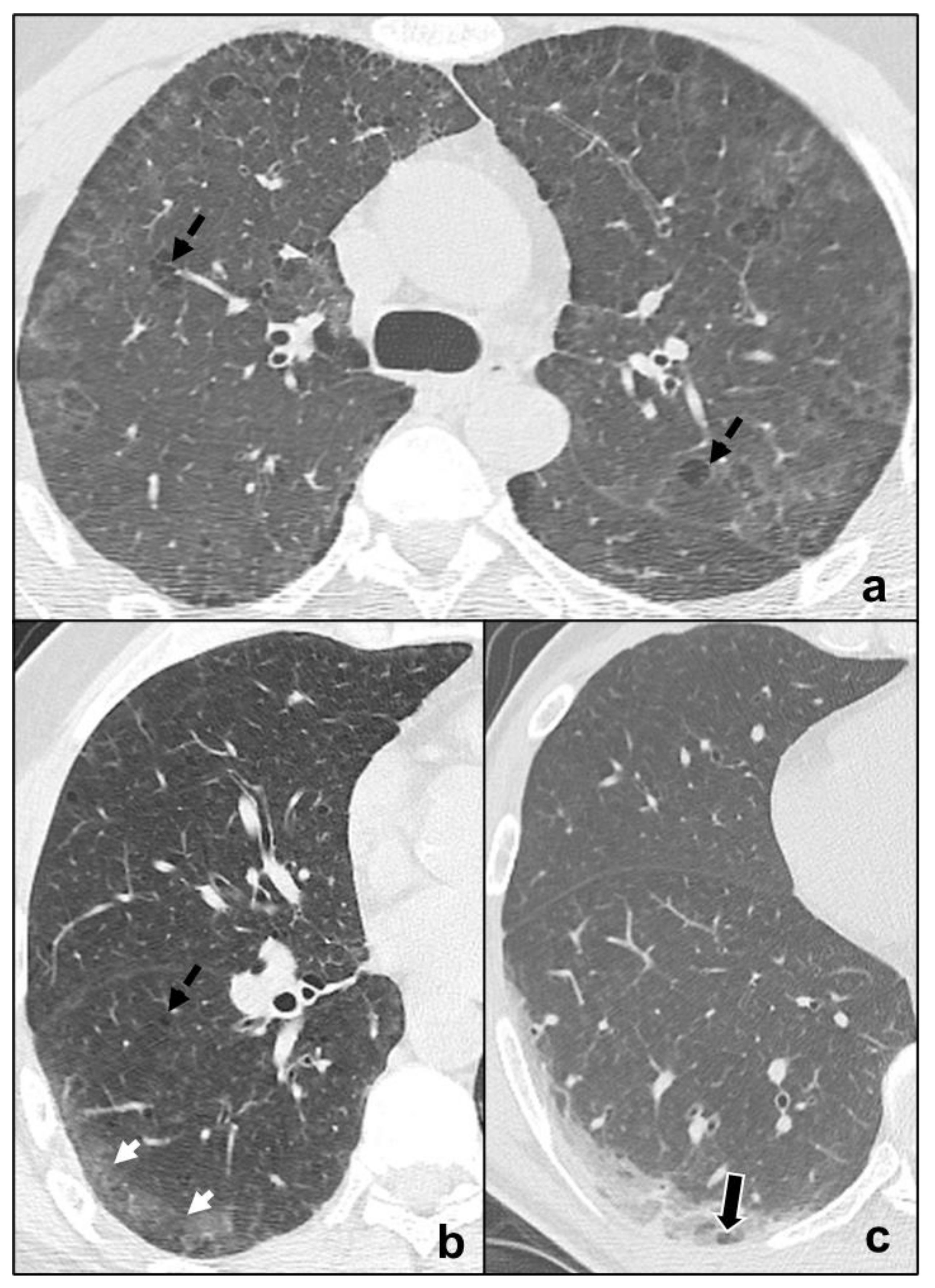
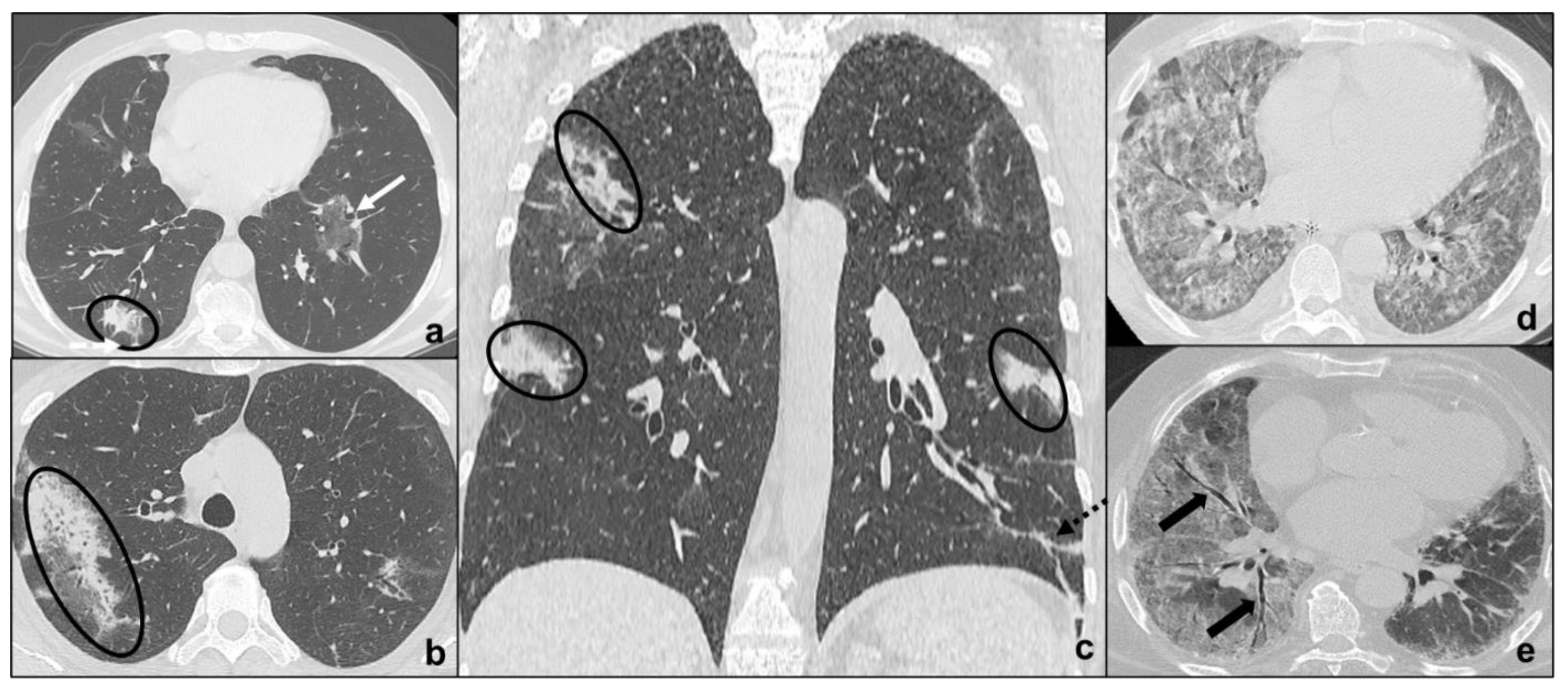
tendency of the consolidations to migrate (OP) [46] (Figure 5a–c);
-
preferential involvement of lobular periphery, with relative preservation of the lobular core, resulting in a “peri-lobular pattern” (OP) [4] (Figure 5a–c);
-
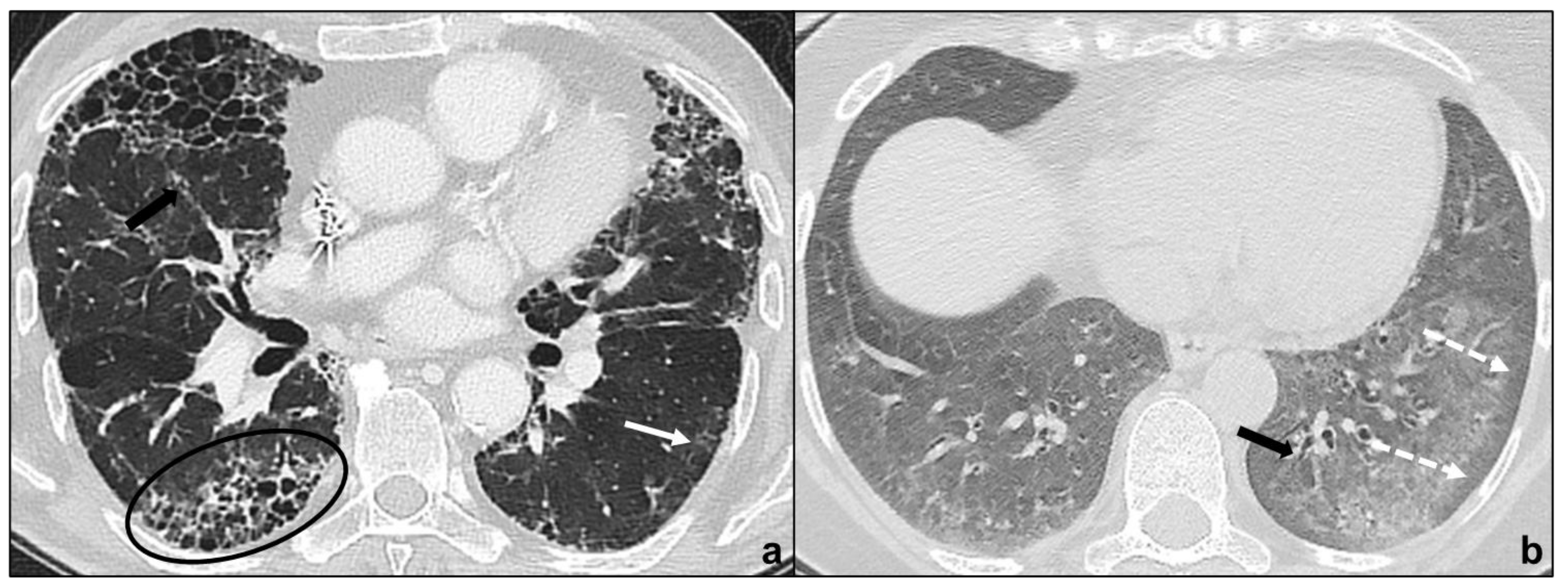
ground-glass opacities during disease exacerbation, but limited in extent (IPF/UIP) [18] (Figure 3a);
-
relative sparing of subpleural pulmonary tissue (NSIP, OP) [18] (Figure 3b and Figure 5a–c);
-
apicobasal gradient and heterogeneous involvement of the lungs (IPF/UIP) [61] (Figure 3a);
-
pleural effusions (exudative AIP, OP) [4] (Figure 5a–d).
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/tomography7030035
References
- Hani, C.; Trieu, N.H.; Saab, I.; Dangeard, S.; Bennani, S.; Chassagnon, G.; Revel, M.-P. COVID-19 pneumonia: A review of typical CT findings and differential diagnosis. Diagn. Interv. Imaging 2020, 101, 263–268.
- Guarnera, A.; Podda, P.; Santini, E.; Paolantonio, P.; Laghi, A. Differential diagnoses of COVID-19 pneumonia: The current challenge for the radiologist-a pictorial essay. Insights Imaging 2021, 12, 34–44.
- Dai, W.-C.; Zhang, H.-W.; Yu, J.; Xu, H.-J.; Chen, H.; Luo, S.-P.; Zhang, H.; Liang, L.-H.; Wu, X.-L.; Lei, Y.; et al. CT Imaging and Differential Diagnosis of COVID-19. Can. Assoc. Radiol. J. 2020, 71, 195–200.
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748.
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A Familial Cluster of Pneumonia Associated with the 2019 Novel Coronavirus Indicating Person-to-Person Transmission: A Study of a Family Cluster. Lancet 2020, 395, 514–523.
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- Pormohammad, A.; Ghorbani, S.; Baradaran, B.; Khatami, A.; Turner, R.; Mansournia, M.A.; Kyriacou, D.N.; Idrovo, J.-P.; Bahr, N.C. Clinical characteristics, laboratory findings, radiographic signs and outcomes of 61,742 patients with confirmed COVID-19 infection: A systematic review and meta-analysis. Microb. Pathog. 2020, 147, 104390.
- Böger, B.; Fachi, M.M.; Vilhena, R.O.; Cobre, A.F.; Tonin, F.S.; Pontarolo, R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am. J. Infect. Control. 2021, 49, 21–29.
- Rubin, G.D.; Ryerson, C.J.; Haramati, L.B.; Sverzellati, N.; Kanne, J.P.; Raoof, S.; Schluger, N.W.; Volpi, A.; Yim, J.-J.; Martin, I.B.K.; et al. The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic: A Multinational Consensus Statement from the Fleischner Society. Radiology 2020, 296, 172–180.
- Wong, H.Y.F.; Lam, H.Y.S.; Fong, A.H.-T.; Leung, S.T.; Chin, T.W.-Y.; Lo, C.S.Y.; Lui, M.M.-S.; Lee, J.C.Y.; Chiu, K.W.-H.; Chung, T.W.-H.; et al. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology 2020, 296, E72–E78.
- Jacobi, A.; Chung, M.; Bernheim, A.; Eber, C. Portable chest X-ray in coronavirus disease-19 (COVID-19): A pictorial review. Clin. Imaging 2020, 64, 35–42.
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes on Chest CT During Recovery from 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology 2020, 295, 715–721.
- Bernheim, A.; Mei, X.; Huang, M.; Yang, Y.; Fayad, Z.A.; Zhang, N.; Diao, K.; Lin, B.; Zhu, X.; Li, K.; et al. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology 2020, 295, 685–691.
- Chung, M.; Bernheim, A.; Mei, X.; Zhang, N.; Huang, M.; Zeng, X.; Cui, J.; Xu, W.; Yang, Y.; Fayad, Z.A.; et al. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology 2020, 295, 202–207.
- Kanne, J.P.; Little, B.P.; Chung, J.H.; Elicker, B.M.; Ketai, L.H. Essentials for Radiologists on COVID-19: An Update—Radiology Scientific Expert Panel. Radiology 2020, 296, E113–E114.
- Sheard, S.; Rao, P.; Devaraj, A. Imaging of acute respiratory distress syndrome. Respir. Care 2012, 57, 607–612.
- Raghu, G.; Remy-Jardin, M.; Myers, J.; Richeldi, L.; Wilson, K.C. The 2018 Diagnosis of Idiopathic Pulmonary Fibrosis Guidelines: Surgical Lung Biopsy for Radiological Pattern of Probable Usual Interstitial Pneumonia Is Not Mandatory. Am. J. Respir. Crit. Care Med. 2019, 200, 1089–1092.
- Mueller-Mang, C.; Grosse, C.; Schmid, K.; Stiebellehner, L.; Bankier, A.A. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics 2007, 27, 595–615.
- Dodd, J.D.; de Jong, P.A.; Levy, R.D.; Coxson, H.O.; Mayo, J.R. Conventional high-resolution CT versus contiguous multidetector CT in the detection of bronchiolitis obliterans syndrome in lung transplant recipients. J. Thorac. Imaging 2008, 23, 235–243.
- Hansell, D.M. Thin-section CT of the lungs: The Hinterland of normal. Radiology 2010, 256, 695–711.
- Kubo, T.; Ohno, Y.; Kauczor, H.U.; Hatabu, H. Radiation dose reduction in chest CT—Review of available options. Eur. J. Radiol. 2014, 83, 1953–1961.
- Braun, F.M.; Johnson, T.R.C.; Sommer, W.H.; Thierfelder, K.M.; Meinel, F.G. Chest CT using spectral filtration: Radiation dose, image quality, and spectrum of clinical utility. Eur. Radiol. 2015, 25, 1598–1606.
- Pontana, F.; Billard, A.-S.; Duhamel, A.; Schmidt, B.; Faivre, J.-B.; Hachulla, E.; Matran, R.; Remy, J.; Remy-Jardin, M. Effect of Iterative Reconstruction on the Detection of Systemic Sclerosis–related Interstitial Lung Disease: Clinical Experience in 55 Patients. Radiology 2016, 279, 297–305.
- De Margerie-Mellon, C.; de Bazelaire, C.; Montlahuc, C.; Lambert, J.; Martineau, A.; Coulon, P.; de Kerviler, E.; Beigelman, C. Reducing Radiation Dose at Chest CT: Comparison Among Model-based Type Iterative Reconstruction, Hybrid Iterative Reconstruction, and Filtered Back Projection. Acad. Radiol. 2016, 23, 1246–1254.
- Mayo, J.R. CT evaluation of diffuse infiltrative lung disease: Dose considerations and optimal technique. J. Thorac. Imaging 2009, 24, 252–259.
- Tokura, S.; Okuma, T.; Akira, M.; Arai, T.; Inoue, Y.; Kitaichi, M. Utility of expiratory thin-section CT for fibrotic interstitial pneumonia. Acta Radiol. 2014, 55, 1050–1055.
- Miller, W.T., Jr.; Chatzkel, J.; Hewitt, M.G. Expiratory air trapping on thoracic computed tomography. A diagnostic subclassification. Ann. Am. Thorac. Soc. 2014, 11, 874–881.
- Kim, M.; Lee, S.M.; Song, J.-W.; Do, K.-H.; Lee, H.J.; Lim, S.; Choe, J.; Park, K.J.; Park, H.J.; Kim, H.J.; et al. Added value of prone CT in the assessment of honeycombing and classification of usual interstitial pneumonia pattern. Eur. J. Radiol. 2017, 91, 66–70.
- Sverzellati, N.; Lynch, D.A.; Hansell, D.M.; Johkoh, T.; King, T.E., Jr.; Travis, W.D. American Thoracic Society-European Respiratory Society Classification of the Idiopathic Interstitial Pneumonias: Advances in Knowledge since 2002. Radiographics 2015, 35, 1849–1871.
- Hunninghake, G.W.; Lynch, D.A.; Galvin, J.R.; Gross, B.H.; Mu¨ller, N.; Schwartz, D.A.; King, T.E.; Lynch, J.P.; Hegele, R.; Waldron, J.; et al. Radiologic Findings Are Strongly Associated with a Pathologic Diagnosis of Usual Interstitial Pneumonia. Chest 2003, 124, 1215–1223.
- Oliveira, D.S.; de Araújo Filho, J.A.; Paiva, A.F.L.; Ikari, E.S.; Chate, R.C.; Nomura, C.H. Idiopathic interstitial pneumonias: Review of the latest American Thoracic Society/European Respiratory Society classification. Radiol. Bras. 2018, 51, 321–327.
- Akira, M.; Inoue, Y.; Kitaichi, M.; Yamamoto, S.; Arai, T.; Toyokawa, K. Usual interstitial pneumonia and nonspecific interstitial pneumonia with and without concurrent emphysema: Thin-section CT findings. Radiology 2009, 251, 271–279.
- Souza, H.A.P.H.M.; Nogueira, K.S.; Matos, A.P.; Vieira, R.P.; Riedi, C.A.; Rosário, N.A.; Telles, F.Q.; Costa, L.M.D. Early microbial colonization of cystic fibrosis patients identified by neonatal screening, with emphasis on Staphylococcus aureus. J. Pediatr. 2006, 82, 377–382.
- Johkoh, T.; Müller, N.L.; Colby, T.V.; Ichikado, K.; Taniguchi, H.; Kondoh, Y.; Fujimoto, K.; Kinoshita, M.; Arakawa, H.; Yamada, H.; et al. Nonspecific interstitial pneumonia: Correlation between thin-section CT findings and pathologic subgroups in 55 patients. Radiology 2002, 225, 199–204.
- Suda, T.; Kono, M.; Nakamura, Y.; Enomoto, N.; Kaida, Y.; Fujisawa, T.; Imokawa, S.; Yasuda, K.; Hashizume, H.; Yokomura, K.; et al. Distinct prognosis of idiopathic nonspecific interstitial pneumonia (NSIP) fulfilling criteria for undifferentiated connective tissue disease (UCTD). Respir. Med. 2010, 104, 1527–1534.
- Silva, C.I.S.; Müller, N.L.; Lynch, D.A.; Curran-Everett, D.; Brown, K.K.; Lee, K.S.; Chung, M.P.; Churg, A. Chronic hypersensitivity pneumonitis: Differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology 2008, 246, 288–297.
- Sieminska, A.; Kuziemski, K. Respiratory bronchiolitis-interstitial lung disease. Orphanet J. Rare Dis. 2014, 9, 1–7.
- Park, J.S.; Brown, K.K.; Tuder, R.M.; Hale, V.A.E.; King, T.E., Jr.; Lynch, D.A. Respiratory bronchiolitis-associated interstitial lung disease: Radiologic features with clinical and pathologic correlation. J. Comput. Assist. Tomogr. 2002, 26, 13–20.
- Akira, M.; Yamamoto, S.; Hara, H.; Sakatani, M.; Ueda, E. Serial computed tomographic evaluation in desquamative interstitial pneumonia. Thorax 1997, 52, 333–337.
- Kawabata, Y.; Hoshi, E.; Murai, K.; Ikeya, T.; Takahashi, N.; Saitou, Y.; Kurashima, K.; Ubukata, M.; Takayanagi, N.; Sugita, H.; et al. Smoking-related changes in the background lung of specimens resected for lung cancer: A semiquantitative study with correlation to postoperative course. Histopathology 2008, 53, 707–714.
- Katzenstein, A.-L.A.; Mukhopadhyay, S.; Zanardi, C.; Dexter, E. Clinically occult interstitial fibrosis in smokers: Classification and significance of a surprisingly common finding in lobectomy specimens. Hum. Pathol. 2010, 41, 316–325.
- Snider, G.L.; Kleinerman, J.; Thurlbeck, W.M.; Bengali, Z.H. The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am. Rev. Respir. Dis. 1985, 132, 182–185.
- Cordier, J.F. Organising pneumonia. Thorax 2000, 55, 318–328.
- Kim, S.J.; Lee, K.S.; Ryu, Y.H.; Yoon, Y.C.; Choe, K.O.; Kim, T.S.; Sung, K.J. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: Diagnostic implications. AJR Am. J. Roentgenol. 2003, 180, 1251–1254.
- Cordier, J.-F. Cryptogenic organising pneumonia. Eur. Respir. J. 2006, 28, 422–446.
- Izumi, T.; Kitaichi, M.; Nishimura, K.; Nagai, S. Bronchiolitis obliterans organizing pneumonia. Clinical features and differential diagnosis. Chest 1992, 102, 715–719.
- Cordier, J.-F.; Loire, R.; Brune, J. Idiopathic Bronchiolitis Obliterans Organizing Pneumonia. Chest 1989, 96, 999–1004.
- Akira, M.; Yamamoto, S.; Sakatani, M. Bronchiolitis obliterans organizing pneumonia manifesting as multiple large nodules or masses. Am. J. Roentgenol. 1998, 170, 291–295.
- Haro, M.; Vizcaya, M.; Texidó, A.; Aguilar, X.; Arevalo, M. Idiopathic bronchiolitis obliterans organizing pneumonia with multiple cavitary lung nodules. Eur. Respir. J. 1995, 8, 1975–1977.
- Li, X.-R.; Peng, S.-C.; Wei, L.-Q. Nonspecific interstitial pneumonia overlaps organizing pneumonia in lung-dominant connective tissue disease. Int. J. Clin. Exp. Pathol. 2015, 8, 11230–11235.
- Johkoh, T.; Müller, N.L.; Taniguchi, H.; Kondoh, Y.; Akira, M.; Ichikado, K.; Ando, M.; Honda, O.; Tomiyama, N.; Nakamura, H. Acute interstitial pneumonia: Thin-section CT findings in 36 patients. Radiology 1999, 211, 859–863.
- Ichikado, K.; Suga, M.; Muranaka, H.; Gushima, Y.; Miyakawa, H.; Tsubamoto, M.; Johkoh, T.; Hirata, N.; Yoshinaga, T.; Kinoshita, Y.; et al. Prediction of prognosis for acute respiratory distress syndrome with thin-section CT: Validation in 44 cases. Radiology 2006, 238, 321–329.
- Ichikado, K.; Johkoh, T.; Ikezoe, J.; Takeuchi, N.; Kohno, N.; Arisawa, J.; Nakamura, H.; Nagareda, T.; Itoh, H.; Ando, M. Acute interstitial pneumonia: High-resolution CT findings correlated with pathology. AJR Am. J. Roentgenol 1997, 168, 333–338.
- Ichikado, K.; Suga, M.; Müller, N.L.; Taniguchi, H.; Kondoh, Y.; Akira, M.; Johkoh, T.; Mihara, N.; Nakamura, H.; Takahashi, M.; et al. Acute interstitial pneumonia: Comparison of high-resolution computed tomography findings between survivors and nonsurvivors. Am. J. Respir. Crit. Care Med. 2002, 165, 1551–1556.
- Rice, A.J.; Wells, A.U.; Bouros, D.; du Bois, R.M.; Hansell, D.M.; Polychronopoulos, V.; Vassilakis, D.; Kerr, J.R.; Evans, T.W.; Nicholson, A.G. Terminal diffuse alveolar damage in relation to interstitial pneumonias. An autopsy study. Am. J. Clin. Pathol. 2003, 119, 709–714.
- Katzenstein, A.L.; Fiorelli, R.F. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am. J. Surg. Pathol. 1994, 18, 136–147.
- Collard, H.R.; Moore, B.B.; Flaherty, K.R.; Brown, K.K.; Kaner, R.J.; King, T.E., Jr.; Lasky, J.A.; Loyd, J.E.; Noth, I.; Olman, M.A.; et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 636–643.
- Akira, M.; Kozuka, T.; Yamamoto, S.; Sakatani, M. Computed Tomography Findings in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2008, 178, 372–378.
- Honda, O.; Johkoh, T.; Ichikado, K.; Tomiyama, N.; Maeda, M.; Mihara, N.; Higashi, M.; Hamada, S.; Naito, H.; Yamamoto, S.; et al. Differential diagnosis of lymphocytic interstitial pneumonia and malignant lymphoma on high-resolution CT. AJR Am. J. Roentgenol. 1999, 173, 71–74.
- Frankel, S.K.; Cool, C.D.; Lynch, D.A.; Brown, K.K. Idiopathic pleuroparenchymal fibroelastosis: Description of a novel clinicopathologic entity. Chest 2004, 126, 2007–2013.
- Chandler, P.W.; Shin, M.S.; Friedman, S.E.; Myers, J.L.; Katzenstein, A.L. Radiographic manifestations of bronchiolitis obliterans with organizing pneumonia vs usual interstitial pneumonia. AJR Am. J. Roentgenol. 1986, 147, 899–906.
