The concomitant hydrolysis and dehydration of biomass-derived cellulose and hemicellulose to furfural (FUR) and 5-(hydroxymethyl)furfural (HMF) under acid catalysis allows a dramatic reduction in the oxygen content of the parent sugar molecules with a 100% carbon economy. However, most applications of FUR or HMF necessitate synthetic modifications. Catalytic hydrogenation and hydrogenolysis have been recognized as efficient strategies for the selective deoxygenation and energy densification of biomass-derived furfurals generating water as the sole byproduct.
- lignocellulose
- renewable chemistry
- catalysis
- furanic biofuels
- hydrogenation
- hydrogenolysis
- carbohydrate
- furfural
- 5-(hydroxymethyl)furfural
1. Introduction
Adopting biomass-derived fuels and chemicals would help relieve the economic and environmental distresses triggered by the excessive use of petrofuels and petrochemicals [1]. Furthermore, the suitable integration of renewable, carbon-neutral biomass in the chemical industry would be an indispensable step toward their long-anticipated sustainability [2][3]. However, selecting the biomass feedstock is critical to not compete with the animal and human food supply chain [4]. In this regard, cellulosic biomass has been considered as a suitable feedstock to produce fuels and chemicals [5]. Only a fraction of around 180 billion tons of lignocellulosic biomass produced annually in the world is utilized [6]. The major challenge in the value addition of biomass is to selectively depolymerize, defunctionalize and deoxygenate the biopolymers into simple molecules with desired structural features and physicochemical properties [7][8]. In this regard, the catalytic value addition of biomass is advantageous since the processes are fast, selective, biomass independent and the chemical industries are well-versed with the catalytic processes [9][10]. The acid-catalyzed hydrolysis of cellulose and lignocellulose fractions into sugars followed by dehydration of the latter into furanics such as furfural (FUR) and 5-(hydroxymethyl)furfural (HMF) are well-documented in the literature [11]. The sequential elimination of three molecules of water from a molecule of glucose forms HMF under acid catalysis [12]. The elegant process decreases the oxygen content of glucose by 50 mol% without cleaving C-C bonds or producing toxic byproducts. Similarly, the acid-catalyzed dehydration of xylose, a major constituent sugar in the pentosans, produces FUR [13]. Both FUR and HMF can access various synthetic value addition pathways with several established and emerging commercial markets for their derivatives [11]. The removal of all the oxygen atoms from a mole of glucose by combining catalytic hydrogenation and hydrogenolysis reactions requires seven moles of hydrogen and leads to n-hexane ( Scheme 1 ) [14]. The process can be contemplated as a renewable production of naphtha-range hydrocarbons. On the other hand, the combination of acid-catalyzed dehydration and catalytic hydrogenation of glucose can lead to more valuable furanic chemicals via the FUR and HMF intermediates [15][16]. The value addition of cellulosic biomasses via the furanic platform allows to preserve some of the crucial functionalities in the sugar moiety and exploit them for synthetic upgrading.
The source of molecular hydrogen for the catalytic reduction of furfurals is of utmost importance. At present, hydrogen is commercially produced by the steam reforming of natural gas [17]. Petroleum and coal are the other two major sources of hydrogen production [18]. In recent years, hydrogen production via the electrolysis of water is gaining more interest and is expected to increase its market share at an accelerated pace [19].
Hydrogen gas produced by polluting technologies is termed ‘grey hydrogen’ and does not have appreciable environmental benefits in their applications [20]. Hydrogen produced from renewable resources such as the electrolysis of water by producing electricity from a combination of renewable energy sources (e.g., solar, hydro) is termed ‘green hydrogen’ and is preferred for its environmental incentives [21]. Other color codes of hydrogen gas include ‘yellow hydrogen’ where solar energy is used alone for electricity generation, and ‘pink hydrogen’ using nuclear energy. There is also ‘blue hydrogen’, where it is produced by the steam reforming of natural gas, but the CO 2 is captured, stored permanently and not allowed to release into the environment. Biomass can be converted into hydrogen by using thermal routes, such as gasification and steam-reforming of biomass feedstock, and enzymatic routes such as fermentative hydrogen production and biocatalyzed electrolysis [22][23][24]. The chemocatalytic conversion of biomass to hydrogen is an emerging field [25]. Sourcing molecular hydrogen and furfurals for downstream energy densification could prove a self-reliant and sustainable chemical technology and circular economy. Converting sugars such as glucose into molecular hydrogen by electrocatalysis has received significant interests in recent years. Biomass-derived hydrogen can be made cleaner than ‘grey hydrogen’ depending on the feedstock chosen, technology adopted and utilization of the byproduct, i.e., CO 2. Finally, it may be said that until the production of hydrogen by electrolysis of water using renewable energy sources reaches technical maturity, a combination of the above technologies is the near future to satisfy the increasing demand of hydrogen.
Catalytic transfer hydrogenation (CTH) is increasingly being used for biomass value addition pathways [26]. The CTH processes use a liquid hydrogen donor molecule (alone or diluted in an inert solvent) in the presence of a suitable metal-based catalyst. 2-Propanol, 2-butanol, ethanol and methanol are commonly used alcoholic hydrogen donor molecule that produces molecular hydrogen in the presence of a metal catalyst (e.g., Pd, Ru) and become oxidized into their corresponding aldehydes or ketones. Formic acid, formate salts and cyclohexene are frequently used hydrogen donor molecules. The CTH processes have the advantage of not using overpressure of gaseous hydrogen, thereby working under milder reaction conditions and simplified reactor design. The process uses renewable ‘green hydrogen’ to work. However, the aldehydes or ketones produced must be separated from the product and recycled. In addition, the reaction often requires elevated temperatures for faster dehydrogenation reaction of the hydrogen donor and also basic additives. Often a combination of a hydrogen donor molecule and H 2 gas is used in combination to avoid forming the oxidized product of the former. Even though CTH process is relatively new in biomass value addition, it has produced some remarkable results in producing furanic biofuels (discussed in the later sections). However, the obligation of using a large excess of the hydrogen donor molecules and their energy-intensive separation from the product must be resolved. Further studies on developing robust, inexpensive, efficient and recycle catalysts that allow both the dehydrogenation and hydrogenation reaction to take place on their surface are required.
2. Catalytic Reduction of Furfurals to Furanic Compounds
Dong et al. studied the influence of preparatory methods of the Cu/SiO 2 catalyst to convert FUR into 2MeF. The Cu/SiO 2 catalyst synthesized via the ammonia evaporation method afforded 2MeF in a 95% yield, noticeably higher than the catalyst prepared by the conventional method. The cooperative effect between surface Cu 0, Cu + species and acid sites helped to improve the selectivity of 2MeF [27]. Using the Cu/SiO 2 catalyst prepared by hydrothermal method and methanol as a hydrogen donor solvent, 2MeF was obtained in a 90% yield starting from FUR [28]. Xylose was converted to FAL and then to 2MeF in a continuous fixed bed reactor using a combination of Hβ zeolite and CuO/ZnO/Al 2O 3 catalyst. GBL/water system served as the biphasic solvent system, and the yield of 2MeF increased up to 86.8% due to the combined effect between Hβ zeolite and GBL [29]. Yang et al. synthesized two mineral-derived Cu/ZnO catalysts consisting of aurichalcite and zincian malachite for the hydrogenation of FUR to FAL and 2MeF. The catalyst composed of aurichalcite showed excellent selectivity ( ca. 94.5%) toward 2MeF due to high surface area and high dispersion of Cu species on the catalyst surface [30]. Up to 95% selectivity toward 2MeF was obtained using a 5% Ir/C catalyst under optimized reaction conditions. The catalyst was recovered up to four consecutive cycles without any significant loss of activity [31]. Jaatinen et al. studied the liquid-phase hydrogenation of FUR to 2MeF using monometallic and bimetallic catalysts consisting of Cu, Fe and Ni. With varying metal loading of the prepared catalysts, 10% Ni/C was found to provide the maximum yield of 2MeF [32]. Spinel ferrite supported Ru catalyst prepared via sol-gel method attained 97% conversion of FUR under mild reaction conditions. The catalyst was easily recoverable, and the activity remained unaltered even after five consecutive cycles [33]. A series of Cu-based catalysts were synthesized by complexing copper (II) ions with dodecylamine for the vapor-phase hydrogenation of FUR. Among the synthesized catalysts, 10% Cu catalysts showed better selectivity toward 2MeF at a hydrogen flow rate of 10 mL/min. The use of mesoporous silica as the support provided weak acidic sites for the hydrogenolysis of C–O bond in FAL [34]. Interestingly, a self-supported nanoporous Cu-Al-Co ternary alloy catalyst developed by Hutchings et al. exhibited better catalytic activity and stability toward the HDO of FUR [35]. A Cu catalyst supported on activated carbon efficiently converted FUR to 2MeF via the FAL intermediate. The catalyst calcinated at 400 °C for 2 h afforded a quantitative yield of 2MeF [36]. Wang et al. studied the effect of acidity on the catalytic performance of Ni 2P in liquid-phase HDO of FUR to 2MeF. The synergistic effect between Brønsted and Lewis acidic sites present in the catalyst increased the catalytic efficiency resulting in the quantitative conversion of FUR [37]. A Cu-Cu 2O/N-RGO catalyst, prepared via microwave-assisted reduction and ammonia evaporation, also possessed high catalytic activity [38]. A bifunctional, mesoporous Cu-Al 2O 3 catalyst exhibited enhanced reactivity toward HDO of FUR due to its high dispersion and small particle size. The Lewis acid sites present on the oxide support are highly electrophilic and interact with the formyl group in FUR [39]. In the two catalysts mentioned above, the catalytic activity is attributed to the synergistic effects of surface Cu 0 and Cu + species in the catalyst. Vapor-phase HDO of FUR using molybdenum carbide catalyst at low temperature and pressure exhibited selectivity toward 2MeF and furan [40]. The density functional theory (DFT) calculations, surface science experiments and flow-reactor evaluation revealed that molybdenum carbide is a promising catalyst for the HDO of FUR [41]. Methanol acted as the hydrogen donor for the CTH of FUR to 2MeF in the presence of a FeVO 4 catalyst. DMF and 2-vinylfuran were identified as the byproducts during the reaction [42]. The Pt and Ru-based noble-metal catalysts afforded quantitative conversion of FUR even at relatively low metal loading (ca. 3 wt.%). The superior activity of the catalysts was attributed to high metal dispersions on the supporting material. In comparison, the Ni-based catalysts had lesser activity toward the hydrogenation reaction. The particle size of Pt was estimated in the range of 5–10 nm, whereas the particle size was below 5 nm in Ru catalysts [43]. Dong et al. studied the effect of catalyst supports (SiO 2, Al 2O 3 and ZnO) on the synthesis of 2MeF using chromium-free Cu catalysts. The Cu/SiO 2 catalyst exhibited the best activity, which accredits to the adsorption and desorption behavior of the catalyst and the presence of weak acidic sites on its surface. Cu/Al 2O 3 and Cu/ZnO catalysts exhibited low selectivity toward 2MeF but high selectivity for FAL, respectively [44]. Dehydration of xylose into FUR was carried out in a plug-flow reactor under mild reaction conditions in a biphasic solvent system, then hydrogenated to 2MeF using a Cu/Fe catalyst. A noticeable decrease in catalytic activity was observed after 20 h of reaction time [45]. Electrochemical hydrogenation of FUR to 2MeF resulted in 60% faradaic efficiency using phosphorus-doped carbon-supported single atom Cu catalyst. The catalyst was synthesized by pyrolyzing chitosan and coordinating it with Cu 2+ ions [46].
Several bimetallic catalysts were used for the hydrogenation of FUR, which furnished 2MeF in good yields. Different supporting materials were synthesized for the Cu-Co bimetallic catalysts (e.g., Cu–Co/SiO 2, Cu–Co/γ-Al 2O 3 and Cu–Co/H-ZSM-5) with varying Cu/Co molar ratios for the liquid-phase hydrogenation of FUR to 2MeF. The catalyst supported on acidic carriers (H-ZSM-5 and γ-Al 2O 3) were found to be successful toward the hydrogenation reaction due to the formation of spinel CuCo 2O 4 oxides. Out of the three catalysts, the Cu–Co/γ-Al 2O 3 (Cu/Co = 1) catalyst exhibited the best catalytic performance due to the presence of additional Cu-CoO x species [47]. Formic acid was used as the hydrogen donor to synthesize 2MeF in a 92% yield over the 10% Ni-Cu/Al 2O 3 catalyst. At high Ni loadings, a gradual decrease in the acidity of catalyst was observed due to less availability of support surface [48]. Dohade et al. reported a 59% yield of 2MeF using a Pt-Co/C catalyst under mild reaction conditions. The catalyst was recycled and reused for four consecutive cycles without any significant loss of activity [49]. A combined yield of 83.9% (2MeF and MTHF) was achieved using the Cu-Pd/ZrO 2 catalyst [50]. Gandarias et al. reported an innovative approach for the production of 2MeF from corncob, which involved the production of FUR and its selective conversion to 2MeF over the Cu-Co/γ-Al 2O 3 [51]. Carbon-supported Ni-Cu catalyst formed 2MeF in a 91% yield at a reaction temperature of 200 °C. Formic acid as a hydrogen source increased the conversion of FUR to 2MeF in 2-propanol [52]. Similarly, alumina-supported Cu-Ni catalyst synthesized by coprecipitation method effectively catalyzed the conversion of FUR to 2MeF and MTHF. A combined yield of 85% for 2MeF and MTHF was obtained under the optimized reaction conditions in the presence of 2-propanol [53]. The Cu-Re/Al 2O 3 catalyst attained complete conversion of FUR at 220 °C and 4 h in 2-propanol. The high stability of the catalyst is attributed to the synergism between the support and metal nanoparticles (NPs). The Cu and Ru species enhanced the hydrogenation and hydrogenolysis of FUR and FAL, respectively [54].
A major concern about the catalytic hydrogenation processes is the recovery and recyclability of the metal catalyst used. Very low mol% of active metal should be used, especially for catalysts involving the noble metals. Ideally, the catalyst should be reusable for multiple cycles without requiring extensive reactivation steps (e.g., calcination). Common deactivation mechanisms of the catalyst candidates include physical blockage of the active sites by coke formation. In addition, chemisorption of various side products on the catalyst surface also deactivates the catalyst. The metal NPs often agglomerate under the reaction conditions forming larger particles with lower surface area available for catalysis. The metal NPs often leach from the catalytic support and enter into the reaction medium. This process not only leads to catalytic deactivation but also product contamination. Even though transition metals are preferably for their low cost and lower environmental footprint, emphasis must be given on the energy requirement of the process since in many cases, the transition metal-based catalysts require more demanding reaction conditions than the noble metal catalysts.
Even though satisfactory results were obtained in some studies, more study is needed in fine-tuning the features of catalysts to improve the selectivity toward DMF. Bimetallic catalysts proved to be efficient for the above conversion, but their stability and recyclability must be considered. The reaction temperature in the range of 180–200 °C and 0.5–3 MPa H 2 pressure were optimal for the synthesis of DMF. High metal dispersion and moderate acidity are the two crucial factors determining the conversion of HMF to DMF.
3. Effect of Process Parameters on The Selectivity of Furanics
Catalytic hydrogenation of the aldehyde group and the furan ring did not require high reaction temperatures. Therefore, the catalytic transformation of FUR and HMF to THFAL and BHMF could be achieved at reaction temperatures around 100 °C. On the other hand, hydrogenolysis of the C–O bond is promoted at a higher temperature. The preparation of 2MeF and DMF typically requires a reaction temperature range of 100–200 °C. Synthesis of 2MeF and DMF above 200 °C was also reported in the literature ( Figure 1 A). Both noble and non-noble catalysts were found effective for the hydrogenation and hydrogenolysis steps. Among the noble metal catalysts, palladium and platinum are the most commonly used, whereas copper and nickel are the most popular non-noble-metal catalysts for the catalytic hydrogenation of furanic compounds ( Figure 1 B). The copper-based catalysts were found effective for the hydrogenation of aldehyde and the hydrogenolysis of C–O bond and used extensively to prepare 2MeF from FUR. Palladium catalysts were also effective for the hydrogenolysis reaction and used for making DMF from HMF. Palladium and nickel-based catalysts were effective for the hydrogenation of furan rings and favored the formation of THFAL from FUR. The ruthenium-based catalysts were also effective for reducing the carbonyl group and used to prepare DMF from HMF. The solvent used in the hydrogenation reactions greatly influences selectivity toward a specific furanic product. Solvent influences the hydrogen bonding of substrate and solubility of H 2 gas. Alcohols are routinely used as a green solvent for catalytic hydrogenation reactions. In some cases, the alcohol solvent also acts as the hydrogen donor. 2-Propanol is the most frequently employed hydrogen-donor alcohol for CTH due to its bulk availability, inexpensiveness, recyclability and low toxicity. Water is also a frequently used solvent for catalytic hydrogenation reactions. The insolubility of many hydrogenated furanic compounds in water allows isolating the products by simple phase separation without employing an organic extractant. Ethers such as 1,4-dioxane have also found use in the hydrogenation reaction ( Figure 1 C). The H 2 pressure in the range of 1–2 MPa was proved as optimal for the hydrogenation of FUR and HMF ( Figure 1 D). The requirement of H 2 pressure is very much dependent on the type of metal catalyst employed. Noble-metal catalysts typically require lesser hydrogen pressure compared to non-noble metal catalysts. In general, the hydrogenation of aldehyde to a hydroxymethyl can be carried out using 1 MPa or less.
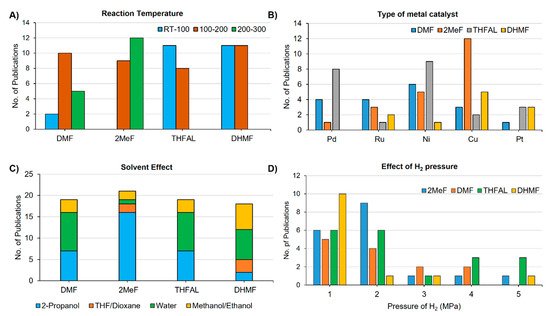
However, hydrogenolysis of the hydroxymethyl group to a methyl group or ring-hydrogenation requires higher pressure (1–2 MPa).
Many of the properties of furanic biofuels closely resemble petrofuels such as gasoline. Table 5 lists the physicochemical and thermal properties of selected furanic biofuels. Among the four compounds, DMF shows superior properties for fuel applications. For example, DMF has the highest calorific value and research octane number, whereas it has least solubility in water.
| Properties | DMF | DMTHF | 2MeF | MTHF |
|---|---|---|---|---|
| Molecular formula/Molar mass | C6H8O/96.13 | C6H12O/100.16 | C5H6O/82.10 | C5H10O/86.13 |
| Density (g/cc) | 0.89 | 0.83 | 0.91 | 0.85 |
| Flash point (°C) | −1 | −26.6 | −22 | −10 |
| Lower calorific value (LCV) (MJ/kg) | 33.8 | 32.8 | 31.2 | 32.8 |
| Research octane number | 119 | 92 | 103 | 86 |
| Solubility in water (g/L) | 1.47 | 6.70 | 3 | 150 |
| (C + H)/O (weight ratio) [a] | 5.00 | 5.25 | 4.12 | 4.37 |
[a] Calculated from the molecular formula.
4. Future Perspectives
The use of abundant, non-food, inexpensive and preferably waste biomass for the production of FUR and HMF is preferred from both the economic as well as environmental perspectives. The problems associated with the isolation of HMF from the reaction media must be sorted out by employing energy-efficient and eco-friendly processes. The hydrophobic analogs of HMF, such as CMF, have shown promise in this regard. However, detailed research is required for toxicological study and life cycle analysis of the biomass-derived furanic compounds to better understand the potential environmental impact in their large-scale use. Newer applications and markets for the furanic compounds must be explored for the preparation to be economically more advantageous. Even though hydrogen can be produced from greener routes such as electrolysis of water and from biomass, the processes yet to reach maturity and commercialization. An inexpensive and sustainable source of hydrogen to synthesize partially-hydrogenated furanic compounds must be ensured for the processes to be considered truly green. The international and national policies regarding the use of biofuels and biorenewable chemicals will continue to play pivotal roles in the commercial adoption of these molecules.
This entry is adapted from the peer-reviewed paper 10.3390/suschem2030029
References
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from Biomass: Technological versus Environmental Feasibility. A Review. Biofuels Bioprod. Biorefin. 2017, 11, 195–214.
- Höfer, R.; Bigorra, J. Biomass-Based Green Chemistry: Sustainable Solutions for Modern Economies. Green Chem. Lett. Rev. 2008, 1, 79–97.
- Philp, J.C.; Ritchie, R.J.; Allan, J.E.M. Biobased Chemicals: The Convergence of Green Chemistry with Industrial Biotechnology. Trends Biotechnol. 2013, 31, 219–222.
- Thompson, P. The Agricultural Ethics of Biofuels: The Food vs. Fuel Debate. Agriculture 2012, 2, 339–358.
- Matson, T.D.; Barta, K.; Iretskii, A.V.; Ford, P.C. One-Pot Catalytic Conversion of Cellulose and of Woody Biomass Solids to Liquid Fuels. J. Am. Chem. Soc. 2011, 133, 14090–14097.
- Langholtz, M.H.; Stokes, B.J.; Eaton, L.M. Energy, U.D. of 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks; ORNL/TM–2016/160; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016; p. 448.
- Maity, S.K. Opportunities, Recent Trends and Challenges of Integrated Biorefinery: Part I. Renew. Sustain. Energy Rev. 2015, 43, 1427–1445.
- Fernando, S.; Adhikari, S.; Chandrapal, C.; Murali, N. Biorefineries: Current Status, Challenges, and Future Direction. Energy Fuels 2006, 20, 1727–1737.
- Geboers, J.A.; Van de Vyver, S.; Ooms, R.; Op de Beeck, B.; Jacobs, P.A.; Sels, B.F. Chemocatalytic Conversion of Cellulose: Opportunities, Advances and Pitfalls. Catal. Sci. Technol. 2011, 1, 714–726.
- Hara, M.; Nakajima, K.; Kamata, K. Recent Progress in the Development of Solid Catalysts for Biomass Conversion into High Value-Added Chemicals. Sci. Technol. Adv. Mater. 2015, 16, 034903.
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a Building Block Platform: Biological Properties, Synthesis and Synthetic Applications. Green Chem. 2011, 13, 754–793.
- Kuster, B.F.M. 5-Hydroxymethylfurfural (HMF). A Review Focussing on Its Manufacture. Starch 1990, 42, 314–321.
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640.
- Liu, S.; Tamura, M.; Nakagawa, Y.; Tomishige, K. One-Pot Conversion of Cellulose into n -Hexane over the Ir-ReOx/SiO2 Catalyst Combined with HZSM-5. ACS Sustain. Chem. Eng. 2014, 2, 1819–1827.
- Caes, B.R.; Teixeira, R.E.; Knapp, K.G.; Raines, R.T. Biomass to Furanics: Renewable Routes to Chemicals and Fuels. ACS Sustain. Chem. Eng. 2015, 3, 2591–2605.
- Verma, S.; Baig, R.B.N.; Nadagouda, M.N.; Len, C.; Varma, R.S. Sustainable Pathway to Furanics from Biomass via Heterogeneous Organo-Catalysis. Green Chem. 2017, 19, 164–168.
- Hydrogen Production: Natural Gas Reforming. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming (accessed on 15 April 2021).
- García, L. Hydrogen production by steam reforming of natural gas and other nonrenewable feedstocks. In Compendium of Hydrogen Energy; Woodhead Publishing: Cambridge, UK, 2015; pp. 83–107.
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. In Proceedings of the Conference Papers in Energy 2013, Limassol, Cyprus, 19–21 November 2012.
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2021, 13, 298.
- Scott, K. Introduction to Electrolysis, Electrolysers and Hydrogen Production. In Electrochemical Methods for Hydrogen Production; Royal Society of Chemistry: London, UK, 2019; pp. 1–27.
- Dou, B.; Zhang, H.; Song, Y.; Zhao, L.; Jiang, B.; He, M.; Ruan, C.; Chen, H.; Xu, Y. Hydrogen Production from the Thermochemical Conversion of Biomass: Issues and Challenges. Sustain. Energy Fuels 2019, 3, 314–342.
- Moreira, F.S.; Machado, R.G.; Romão, B.B.; Batista, F.R.X.; Ferreira, J.S.; Cardoso, V.L. Improvement of Hydrogen Production by Biological Route Using Repeated Batch Cycles. Process Biochem. 2017, 58, 60–68.
- Tanksale, A.; Beltramini, J.N.; Lu, G.M. A Review of Catalytic Hydrogen Production Processes from Biomass. Renew. Sustain. Energy Rev. 2010, 14, 166–182.
- Ni, M.; Leung, D.Y.; Leung, M.K.; Sumathy, K. An Overview of Hydrogen Production from Biomass. Fuel Process. Technol. 2006, 87, 461–472.
- Jin, X.; Yin, B.; Xia, Q.; Fang, T.; Shen, J.; Kuang, L.; Yang, C. Catalytic Transfer Hydrogenation of Biomass-Derived Substrates to Value-Added Chemicals on Dual-Function Catalysts: Opportunities and Challenges. ChemSusChem 2019, 12, 71–92.
- Dong, F.; Ding, G.; Zheng, H.; Xiang, X.; Chen, L.; Zhu, Y.; Li, Y. Highly Dispersed Cu Nanoparticles as an Efficient Catalyst for the Synthesis of the Biofuel 2-Methylfuran. Catal. Sci. Technol. 2016, 6, 767–779.
- Li, B.; Li, L.; Sun, H.; Zhao, C. Selective Deoxygenation of Aqueous Furfural to 2-Methylfuran over Cu0/Cu2O·SiO2 Sites via a Copper Phyllosilicate Precursor without Extraneous Gas. ACS Sustain. Chem. Eng. 2018, 6, 12096–12103.
- Cui, J.; Tan, J.; Cui, X.; Zhu, Y.; Deng, T.; Ding, G.; Li, Y. Conversion of Xylose to Furfuryl Alcohol and 2-Methylfuran in a Continuous Fixed-Bed Reactor. ChemSusChem 2016, 9, 1259–1262.
- Yang, X.; Xiang, X.; Chen, H.; Zheng, H.; Li, Y.-W.; Zhu, Y. Efficient Synthesis of Furfuryl Alcohol and 2-Methylfuran from Furfural over Mineral-Derived Cu/ZnO Catalysts. ChemCatChem 2017, 9, 3023–3030.
- Date, N.S.; Hengne, A.M.; Huang, K.-W.; Chikate, R.C.; Rode, C.V. Single Pot Selective Hydrogenation of Furfural to 2-Methylfuran over Carbon Supported Iridium Catalysts. Green Chem. 2018, 20, 2027–2037.
- Jaatinen, S.K.; Karinen, R.S.; Lehtonen, J.S. Liquid Phase Furfural Hydrotreatment to 2-Methylfuran with Carbon Supported Copper, Nickel, and Iron Catalysts. ChemistrySelect 2017, 2, 51–60.
- Wang, B.; Li, C.; He, B.; Qi, J.; Liang, C. Highly Stable and Selective Ru/NiFe2O4 Catalysts for Transfer Hydrogenation of Biomass-Derived Furfural to 2-Methylfuran. J. Energy Chem. 2017, 26, 799–807.
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Moreno-Tost, R.; Maireles-Torres, P. Selective Production of 2-Methylfuran by Gas-Phase Hydrogenation of Furfural on Copper Incorporated by Complexation in Mesoporous Silica Catalysts. ChemSusChem 2017, 10, 1448–1459.
- Hutchings, G.S.; Luc, W.; Lu, Q.; Zhou, Y.; Vlachos, D.G.; Jiao, F. Nanoporous Cu–Al–Co Alloys for Selective Furfural Hydrodeoxygenation to 2-Methylfuran. Ind. Eng. Chem. Res. 2017, 56, 3866–3872.
- Gong, W.; Chen, C.; Zhang, H.; Wang, G.; Zhao, H. Efficient Synthesis of 2-Methylfuran from Bio-Derived Furfural over Supported Copper Catalyst: The Synergistic Effect of CuOx and Cu. ChemistrySelect 2017, 2, 9984–9991.
- Wang, Y.; Feng, X.; Yang, S.; Xiao, L.; Wu, W. Influence of Acidity on the Catalytic Performance of Ni2P in Liquid-Phase Hydrodeoxygenation of Furfural to 2-Methylfuran. J. Nanopart. Res. 2020, 22, 67.
- Geng, W.; Li, W.; Liu, L.; Liu, J.; Liu, L.; Kong, X. Facile Assembly of Cu-Cu2O/N-Reduced Graphene Oxide Nanocomposites for Efficient Synthesis of 2-Methylfuran. Fuel 2020, 259, 116267.
- Park, S.; Kannapu, H.P.R.; Jeong, C.; Kim, J.; Suh, Y. Highly Active Mesoporous Cu−Al2O3 Catalyst for the Hydrodeoxygenation of Furfural to 2-methylfuran. ChemCatChem 2020, 12, 105–111.
- Lee, W.-S.; Wang, Z.; Zheng, W.; Vlachos, D.G.; Bhan, A. Vapor Phase Hydrodeoxygenation of Furfural to 2-Methylfuran on Molybdenum Carbide Catalysts. Catal. Sci. Technol. 2014, 4, 2340–2352.
- Xiong, K.; Lee, W.-S.; Bhan, A.; Chen, J.G. Molybdenum Carbide as a Highly Selective Deoxygenation Catalyst for Converting Furfural to 2-Methylfuran. ChemSusChem 2014, 7, 2146–2149.
- Grazia, L.; Bonincontro, D.; Lolli, A.; Tabanelli, T.; Lucarelli, C.; Albonetti, S.; Cavani, F. Exploiting H-Transfer as a Tool for the Catalytic Reduction of Bio-Based Building Blocks: The Gas-Phase Production of 2-Methylfurfural Using a FeVO4 Catalyst. Green Chem. 2017, 19, 4412–4422.
- Mäkelä, E.; Lahti, R.; Jaatinen, S.; Romar, H.; Hu, T.; Puurunen, R.L.; Lassi, U.; Karinen, R. Study of Ni, Pt, and Ru Catalysts on Wood-Based Activated Carbon Supports and Their Activity in Furfural Conversion to 2-Methylfuran. ChemCatChem 2018, 10, 3269–3283.
- Dong, F.; Zhu, Y.; Zheng, H.; Zhu, Y.; Li, X.; Li, Y. Cr-Free Cu-Catalysts for the Selective Hydrogenation of Biomass-Derived Furfural to 2-Methylfuran: The Synergistic Effect of Metal and Acid Sites. J. Mol. Catal. A Chem. 2015, 398, 140–148.
- Lessard, J.; Morin, J.-F.; Wehrung, J.-F.; Magnin, D.; Chornet, E. High Yield Conversion of Residual Pentoses into Furfural via Zeolite Catalysis and Catalytic Hydrogenation of Furfural to 2-Methylfuran. Top. Catal. 2010, 53, 1231–1234.
- Zhou, P.; Chen, Y.; Luan, P.; Zhang, X.; Yuan, Z.; Guo, S.-X.; Gu, Q.; Johannessen, B.; Mollah, M.; Chaffee, A.L.; et al. Selective Electrochemical Hydrogenation of Furfural to 2-Methylfuran over a Single Atom Cu Catalyst under Mild pH Conditions. Green Chem. 2021, 23, 3028–3038.
- Srivastava, S.; Jadeja, G.C.; Parikh, J. A Versatile Bi-metallic Copper–Cobalt Catalyst for Liquid Phase Hydrogenation of Furfural to 2-Methylfuran. RSC Adv. 2016, 6, 1649–1658.
- Fu, Z.; Wang, Z.; Lin, W.; Song, W.; Li, S. High Efficient Conversion of Furfural to 2-Methylfuran over Ni-Cu/Al2O3 Catalyst with Formic Acid as a Hydrogen Donor. Appl. Catal. A 2017, 547, 248–255.
- Dohade, M.G.; Dhepe, P.L. One Pot Conversion of Furfural to 2-Methylfuran in the Presence of PtCo Bimetallic Catalyst. Clean Technol. Environ. Policy 2018, 20, 703–713.
- Chang, X.; Liu, A.-F.; Cai, B.; Luo, J.-Y.; Pan, H.; Huang, Y.-B. Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran and 2-Methyltetrahydrofuran over Bimetallic Copper-Palladium Catalysts. ChemSusChem 2016, 9, 3330–3337.
- Gandarias, I.; García-Fernández, S.; Obregón, I.; Agirrezabal-Telleria, I.; Arias, P.L. Production of 2-Methylfuran from Biomass through an Integrated Biorefinery Approach. Fuel Process. Technol. 2018, 178, 336–343.
- Fu, Z.; Wang, Z.; Lin, W.; Song, W. Conversion of Furan Derivatives for Preparation of Biofuels over Ni–Cu/C Catalyst. Energy Sources Part A 2017, 39, 1176–1181.
- Zhang, Z.; Pei, Z.; Chen, H.; Chen, K.; Hou, Z.; Lu, X.; Ouyang, P.; Fu, J. Catalytic In-Situ Hydrogenation of Furfural over Bimetallic Cu–Ni Alloy Catalysts in Isopropanol. Ind. Eng. Chem. Res. 2018, 57, 4225–4230.
- Zhou, K.; Chen, J.; Cheng, Y.; Chen, Z.; Kang, S.; Cai, Z.; Xu, Y.; Wei, J. Enhanced Catalytic Transfer Hydrogenation of Biomass-Based Furfural into 2-Methylfuran over Multifunctional Cu–Re Bimetallic Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 16624–16636.
