Food safety is imperative, especially for infants and young children because of their underdeveloped immune systems. This requires adequate nutritious food with appropriate amounts of macro- and micronutrients. Currently, a well-established system for infant food is enforced by the regulatory bodies, but no clear system exists for complementary food, which is consumed by children from the age of 6 month to 24 months. As the child grows beyond 6 months, the need for nutrients increases, and if the nutritional needs are not fulfilled, it can lead to health problems, such as stunted growth, weak immune system, and cardiovascular diseases. Hence, it is important to have regulatory bodies monitoring complementary food in a similar capacity as is required for infant formula.
1. Introduction
Nutrition at an early stage of life lays a foundation for the optimal growth and development of the human body. Breast milk is the primary food source for children under the age of six months, but as the child grows older, nutrient needs increase. Hence, a complementary food is required to fulfill all nutritional requirements, including energy, proteins, carbohydrates, fats, minerals, and vitamins
[1]. If proper nutrients are not fed according to the child’s age, the child might suffer from problems, such as stunted growth, a weak immune system, cardiovascular diseases, etc. The foods required according to different age groups is illustrated in
Table 1. Complementary food is usually based on staple diets having cereals and legumes as their main ingredient. For a good quality complementary food, there should be sufficiently high protein content, high caloric value per volume of food and adequate amounts of vitamins and minerals
[2]. The other commonly used complementary foods include mashed fruits, vegetables, cooked grains, beans, etc. Currently, there are several commercially prepared complementary food brands available in the market that consist of these ingredients.
Table 1. Different stages of child development and type of food required
[3].
| Category |
Age Group |
Food |
| Neonate |
Birth to 28 days |
Breast Milk/Infant formula |
| Infant |
1 month–6 months |
Breast Milk/Infant formula |
| 6 months–24 months |
Complementary Food |
| Children |
2 to 12 years |
Standard food |
| Adolescent |
12 to 16 years |
Standard food |
In the recent years, issues concerning contamination, fraud and adulteration of infant formula and complementary food have been reported to have increased
[4]. Considering the higher risk of food-borne illness in children, the safety of food during its production, processing and handling practices plays a vital role in a child’s health
[5]. In many countries, there are no specific regulatory requirements for complementary food. It is considered similar to any other food product, because it is regarded as not the sole part of the child’s diet
[6][7]Contamination can occur at numerous points of the food processing stage, i.e., before, during, and after processing. For example, at the point of raw material collection, transportation, cleaning, packaging, etc. Rigorous quality assurance (QA) and quality control (QC) processes can mitigate food safety incidents by having a good understanding of potential sources and routes of contamination
[8]. Quality assurance includes a set of activities to ensure quality in whole production, distribution and procuring raw materials from suppliers safely. Quality control emphasizes the end product. Both processes oversee specific nutrient levels, essential testing for chemical residues, testing of microbiological pathogens and toxins, allergens, intentional and unintentional additives, environmental contaminants, labeling requirements and entire facility operational requirements
[9].
Regulatory bodies are responsible for building laws and regulations to prevent the occurrence of food safety issues
[10]. Countries that do not have the requisite infrastructure to build these laws and regulations follow the international food standards structured by the Food and Agriculture Organization (FAO) and World Health Organization (WHO) under the program known as Codex Alimentarius
[11]. In the United States of America (USA), the food processing sector is controlled by state and federal agencies, wherein Hazard Analysis and Critical Control Point (HACCP) is the management system that helps in controlling microbiological, chemical, and physical hazards as well as improving the quality of products such as meat, poultry, seafood, and juice. Recently, the U.S governing bodies passed the Food and Drug Administration (FDA) Food Safety Modernization Act (FSMA). FSMA aims to help the regulatory bodies prevent the food safety issues instead of just focusing on the aftermath steps.
2. Global Food Safety Standards for Complementary Foods: CODEX Alimentarius & ISO
The Codex Alimentarius defines the standards for follow-up formula as food made from either milk of cows or other animals, or from constituents of plant origin. Preparation should be carried out in a way that prevents any possible spoilage and contamination. The regulation describes that the product should be nutritionally sufficient to promote normal growth of the child with the help of essential nutrient contents of protein, fat, carbohydrates, vitamins, and minerals. All ingredients should be of good quality and appropriate for ingestion. The energy content of 100 mL of product should not be less than 60 kcal and should not be greater than 85 kcal. The essential nutrients required for complementary food are listed in
Table 2. Other than the essential nutrients, these standards also enumerate the amount of food additives, including thickening agents, emulsifiers, pH adjustors, antioxidants, and flavors, as well as the permissible limits of pesticide residue and other contaminants. The standards also specify that after production, appropriate testing of the finished food product should be done in terms of pathogenic microorganisms which may represent hazards to health
[11]. The preparation and packaging should be executed in accordance with the Code of Hygienic Practice for Infants and Young Children (CAC/RCP 66-2008).
Table 2. Essential nutrients listed in Codex standards for follow up formula (complementary food) with minimum and maximum contents. Obtained from
Codex Alimentarius Standard for Follow Up Formula [11].
| Essential Nutrient |
Minimum Content per 100 Available Calories |
Maximum Content per 100 Available Calories |
| Protein |
3 g with nutritional with quality equivalent to or greater than casein. |
5.5 g |
| Fat |
3 g (linolenic acid should not be less than 300 mg per 100 calories) |
6 g |
| Carbohydrates |
Nutritionally available carbohydrates added in accordance to required energy density |
- |
| Vitamins |
| Vitamin A |
167.5 mg |
502.5 mg |
| Vitamin D |
26.8 mg |
80.4 mg |
| Vitamin C (Ascorbic acid) |
8 mg |
- |
| Vitamin B1 (Thiamine) |
40 µg |
- |
| Vitamin B2 |
60 µg |
- |
| Nicotinamide |
250 µg |
- |
| Vitamin B6 |
45 µg |
- |
| Folic Acid |
4 µg |
- |
| Pantothenic acid |
300 µg |
- |
| Vitamin B12 |
0.15 µg |
- |
| Vitamin K1 |
4 µg |
- |
| Biotin |
1.5 µg |
- |
| Minerals |
| Sodium |
20 mg |
85 mg |
| Potassium |
80 mg |
- |
| Chloride |
55 mg |
- |
| Calcium |
90 mg |
- |
| Phosphorous |
60 mg |
- |
| Magnesium |
6 mg |
- |
| Iron |
1 mg |
2 mg |
| Iodine |
5 µg |
- |
| Zinc |
0.5 mg |
- |
The International Organization for Standardization (ISO) is a worldwide organization of national standard bodies. The aim of this organization is to develop standards in cooperation with international industry bodies which are relevant to the market and provide solutions to challenges at the global level. The ISO has strict standards for infant formula, considering its usage for a potentially vulnerable population. The different sections of the regulation mention the range of the essential macro- and micronutrients that should be present in the formula, as well as the appropriate method of quantification, as overviewed in
Table 2 [12]. These quantification methods can also be adapted for complementary food in compliance with the content ranges mentioned in the Codex Alimentarius. Recently, private standards have also emerged and evolved
[13]. The private global food safety standards currently available for the industry are summarized in
Table 3. These private standards are now becoming increasingly important.
Table 3. Examples of private food safety standards for certification for food industries
[13].
| Global Safety Standard Certification |
Description |
| International Organization for Standardization (ISO 22000) |
The ISO 22000 standard describes the food safety management system requirements for any organization involved in the food chain, such as ingredient producers, retailers, catering services, transportation, etc. Any organization can pursue certification and registration if it conforms with this standard. |
| Safe Quality Food (SQF) 1000/2000 |
It is a Hazard Analysis and Critical Control Points (HACCP)-based certification system for food safety and quality of ingredients, packaging, farming, packing houses, etc. |
| British Retail Consortium (BRCGS) |
This standard has been developed in collaboration with the industry for provision of product safety and quality. |
| PrimusGFS |
This certification program is a farm-focused Global Food Safety Initiative (GFSI). |
| Global Good Agricultural Practices (GAP) |
This certification program covers primarily agricultural crops, such as fruits, vegetables, hops, tea, etc. |
| International Featured Standards (IFS) |
This certification program covers the processes in the supply chain by doing risk-based assessments. |
| Food Safety Management Certification (FSSC) 22000 |
This certification is based on ISO 22000, ISO 9001, ISO/TS22003 and ISO 22003 and confirms food safety and quality of the organization certified. |
3. Food Safety Standards for Complementary Food in the United States
The FDA has no specific requirement for complementary food and includes it in general food products. However, it has robust infrastructure and regulations for infant formula, pertaining to its consumption during the critical period of growth of a child. These regulations, if applied to complementary food, can help prevent all types of hazards which have severe consequences for the health of children.
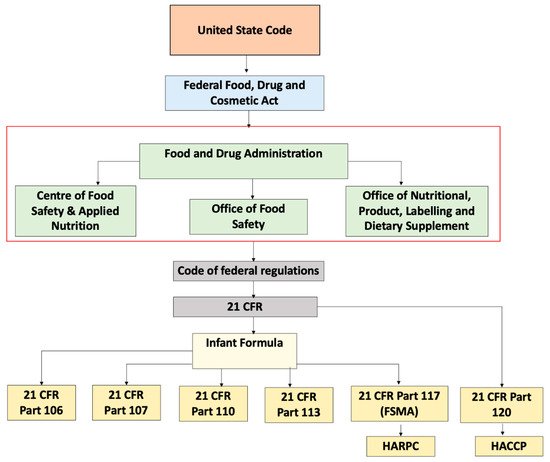
Within FDA, the Center for Food Safety and Applied Nutrition (CFSAN) is accountable for the regulation of infant formula along with the Office of Nutritional Product, Labelling and Dietary Supplements (ONPLDS) and Office of Food Additive Safety (OFAS). The Federal Food Drug Cosmetic Act (FFDCA) is a law that falls under United States Code and gives control to the FDA to manage food, drugs, cosmetics, and medical devices. FFDCA describes infant formula as a food consumed by children less than 12 months old that purports special dietary use for simulation of human milk. Under Section 412 of the FFDCA, prior to the marketing of a new formula, the formula should be registered. Subsequently, the FDA has to be notified if there are any changes in the formula. This section of the FFDCA also describes the responsibility of the FDA to monitor the implementation of all provisions. Every person responsible for either manufacture or distribution of the formula is required to adhere to the requirements stated in the regulation
[14]. The code of federal regulations applicable to the handling of infant formula are shown in
Table 4 and
Figure 1 describes the by law arrangement of these regulations. These existing codes can be used to regulate complementary foods.
Figure 1. Schematic representation of regulations governing infant formula in United States.
Table 4. Code of Federal Regulations (CFR) for the manufacturing of infant formula
[15][16][17][18][19].
| Regulation |
Description |
| 21 CFR Part 106 |
Current Good Manufacturing Practices (cGMP), production and in process control system, prevention of adulteration by workers, facilities, equipment or utensils, ingredients, microorganism, packaging and labelling, audit of cGMP, record keeping, registration, submission and notification. |
| 21 CFR Part 107 |
General provision and applicability, labelling, exemptions, nutrient requirements, recalls, elements of infant formula recall, notification requirements and record retention. |
| 21 CFR Part 110 |
cGMP, packaging, holding of human food, production and process control. |
| 21 CFR Part 113 |
Thermally processed low acid food packaged in hermetically sealed containers. |
| 21 CFR Part 117 |
cGMP, hazard analysis and risk-based preventive controls for human food, requirements applying to records that must be established and maintained and the supply chain program |
| 21 CFR Part 120 |
HACCP, The regulation primarily pertains to juice products. |
Hazard Analysis and Critical Control Point (HACCP) and Food Safety Modernization Act (FSMA)
HACCP and FSMA are the food safety systems that enable the development of efficient controls for the production of food products so that there is a minimal occurrence food-related hazard
[20]. These food safety systems also facilitate traceability and help in auditing the manufacturing process.
HACCP is a management system that addresses food safety from various aspects. It involves addressing food safety issues by analysis of biological, chemical and physical hazards in all sections of food manufacturing, including growing, harvesting, processing, and distributing
[21]. The HACCP plan does not stand alone in a processing facility; it is built on other food safety plans, such as the prerequisite programs, which are designed to provide basic operating conditions for safe production of food, Good Manufacturing Practices (GMP), and sanitation standard operating procedures. The Current Good Manufacturing Practices (cGMPs) cover the in-process control systems, including controls to prevent contamination by workers, facilities, automatic equipment, utensils, ingredients, and containers. cGMPs also oversee adulteration from microorganisms, packaging material, labelling and the release of finished product. The minimum cGMPs that should be used in facilities for manufacturing, processing, packaging and holding of formula to ensure adequate retention of nutrients is also listed in the plan.
The preliminary tasks in development of the HACCP plan involves assembling the team, contemplation about the kind of food and its ingredients, and planning for processing and distribution with respect to its targeted consumer and intended use. Assembling the team involves collecting individuals who have the knowledge and experience with the target food product. These individuals have the responsibility to conduct the hazard analysis, identify potential hazards, suggest the controls and critical limits, frame procedures for monitoring and verification, recommend corrective actions and validate the food safety plan. The identified hazards are further categorized into their likeliness of occurrence, i.e., if they are reasonably likely to occur or non-reasonably likely to occur, so as to have relevant corrective steps associated with each hazard.
The HACCP plan is formulated as a flow diagram to provide a clear outline of the steps in the production process, which includes steps that occur before and after processing. This plan is approved only after on-site review of the operations for verification of accuracy
[22]. Under this system, every procedure and the corresponding obtained data are required for documentation and recordkeeping, which enables the establishment of corrective actions in case of any deviation and verification of the process.
The FSMA was signed on 4 January 2011 with the objective of safeguarding public health by strengthening food safety systems. It gives the FDA new incentives to have the same standards for both imported and domestic food products to build a unified food safety system. It also authorizes preventive controls across the food supply, safety standards for the product, and surveillance of intentional contamination. The law directs the FDA to inspect facilities at regular intervals and to have access to records documenting execution of the food safety plan. It also requires that testing of food products should be done by accredited laboratories to maintain high quality standards. The FSMA also specifies response tools for when problems arise, despite preventive controls, in the form of mandatory recalls and detention of products. The FDA can also suspend the registration of the facility
[23][24]. The FSMA proposes seven major regulations which oversee how produce should be grown, packed, processed, shipped and imported. The regulations emphasize consumer-friendly methods for locating food products in case of recall. The regulations also mandate timely registration of manufacturing facilities and transparency for importers to be informed if their food was rejected by another company. Furthermore, they give authority to FDA to detain suspected food items. The FSMA directs a legislative mandate, wherein elaborative, science-based preventive controls are required across the food supply chain
[25]On 17 September 2015, the FDA declared Hazard Analysis and Risk-based Preventive Controls (HARPC) as a new food safety regulation. HARPC comes under FSMA in the FFDCA. It is also called the FSMA food safety plan. The HARPC regulation enforces preventive controls to identify potential risks or threats to the food supply so that proper corrective steps could be taken instead of fixing critical limits, as was the focus in HACCP. The regulation covers aspects such as manufacturing, processing, storage and corresponds to preventive control management components, monitoring procedures, corrective actions and corrections, verification, validation, and preparation of recall plans. The manufacturing facility is required to have a written record of a monitoring program for regular evaluation, which should be approved by the FDA to make sure there are no inadequacies. Corrective actions should be implemented if the preventive controls are not properly executed if there are doubts regarding the effectiveness of the food safety plan and if there are discrepancies in the implementation of a food safety plan. HARPC also requires food facilities to propose steps to confirm that everything is operating correctly, and a record of everything should be documented if ever a reanalysis is required. HARPC makes the manufacturing process more transparent, with improved control over suppliers, partners and buyers
[26]. The seven steps of HARPC include: assessment of hazards, including product-specific hazards and facility-specific issues; establishment of preventive controls, including sanitation procedures for food contact points, training for hygiene, monitoring of the environment, and authentication of suppliers; monitoring of the efficacy of the controls; establishment of corrective actions and verification methods; recordkeeping and reanalysis of the plan once every 3 years
[27].
The HARPC plan covers food safety concerns beyond CCPs. HARPC relies on aspects such as FDA regulations, standards and guidance documents for preventive control instead of only process controls. All the regulations in FSMA are grounded in HACCP
[28][29]. The differences between HACCP and HARPC have been summarized in
Table 5.
Table 5. Differences between HACCP and HARPC in terms of various elements of food safety plans
[25][27].
| Hazard Analysis and Critical Control Point (HACCP) |
Hazard Analysis and Risk-Based Preventive Controls (HARPC) |
| HACCP is based on the Codex Alimentarius and guidelines given by the National Advisory Committee on Microbiological Criteria for Food. It is required by meat, poultry, seafood and juice industries. |
HARPC is a food safety plan based on the Food Safety Modernization Act (FSMA). |
| HACCP only covers chemical, biological and physical hazards. |
In addition to chemical, biological and physical hazards, HARPC also considers radiological hazards, natural toxins, pesticides and drug residues, parasites, allergens and unapproved food and color additives, and non-intentional and intentional economically motivated hazards. |
| Critical control points are required for the processes. |
Critical control points are required for processes and other points as required for the food safety.
(21CFR117.135) |
| According to HACCP plan, the critical limits are set at CCPs. |
HARPC also has a set of parameters and values in terms of maximum and minimum values. Optimization of these values minimizes the occurrence of hazards. (21CFR117.135 c (1)) |
| HACCP requires all the set process controls to be verified. |
In HARPC, verification is required for all preventive and process controls. Supplier verification is also required. |
| Recall is not required in the plan. |
According to HARPC, a written recall plan is mandatory when a hazard is identified. The written plan should include procedures which explains the aftermath steps and relevant responsibility. |
| The HACCP is required to be reviewed at least once a year or when required. |
The HARPC plan can be reviewed once every three years or when required. |
This entry is adapted from the peer-reviewed paper 10.3390/foods10092199