The surface-modified separator plays a role in improving the electrolyte wettability, homogenizing Li+flux, and strengthening the mechanical/thermal property. Due to these favorable benefits, the formation of sharp Li dendrite is efficiently suppressed and the thermal stability of battery is greatly enhanced. In this article, separator-coating materials are classified into six categories in terms of material characteristics to show how each material has different electrochemical properties. We believe that the suggested approach would become a powerful strategy to improve the performance and stability of next-generation batteries such as lithium-metal batteries.
- Li metal anode
- functional separator
- battery safety
- separator-coating materials
1. Introduction
Since the advent of commercial rechargeable batteries using the combination of graphite and LiCoO 2, secondary lithium-ion batteries (LIBs) as practical energy reservoirs have received continuous attention from industries and academics, owing to their decent energy density, long life span, and low self-discharging rate [1][2][3][4][5][6][7]. As state-of-the-art electronic devices such as electric vehicles (EVs) and portable devices become a big part of our lives, the demand for developing more advanced LIBs with high capacity, fast charging ability, and improved reliability has been accelerated to keep pace with this fast-moving trend [8]. Given the specification of the above-described advanced electronic devices and the mission to reduce greenhouse gas emission to net-zero by 2050, it is inevitable to move on from the LIB technology to more advanced battery systems such as Li metal batteries (LMBs), which can dramatically increase the energy density of the existing energy storage technology [9][10][11]. Before the commercialization of LIBs, Dr. Whittingham first developed a battery prototype using a Li metal paired with a TiS 2 electrode in the 1970s [12]. The developed Li|TiS 2 cell has an electrochemical potential of about 2.5 V, which is not high enough for achieving high specific energy density. In 1989, Moli Energy commercialized Li|MoS 2 cylindrical-type rechargeable batteries that were successful in the commercial market for a while [13]. However, owing to the frequent occurrence of safety issues such as catching fire, LMB products eventually disappeared from the market at the time.
Recently, Li metal anodes have come into the spotlight again due to the limited energy density of existing LIB technology that employ a Li-containing cathode and a carbon-based anode [10][11]. Metallic Li is considered the “holy-grail” anode, owing to its tenfold higher specific capacity than “graphite”, its low material density (0.534 g cm −3 ), and the most negative electrode potential (−3.04 V versus S.H.E. at 25 °C) [10][11]. Furthermore, the adoption of metallic Li as an anode is indispensable to realize high-energy-density batteries such as Li-O 2 and Li-S cells, both of which are being considered the “future of energy storage” [14][15][16][17][18][19][20][21][22][23][24][25]. Due to these promising prospects, a number of research projects focusing on the Li anode have been extensively carried out. Until now, only a few Li anode systems have shown promising results in terms of electrochemical stability and performance. For example, all-solid-state Li metal batteries with a thin Ag-C layer were successfully demonstrated by Samsung’s research group [26]. The designed pouch cell ran over 1000 cycles with maintaining a high energy density ( >900 Wh l −1 ) and an excellent Coulombic efficiency (99.8%). This approach presents a bright outlook where Li-metal systems can be practically adopted as next-generation energy storage. However, there are at least four representative challenges to overcome in order to make all solid-state LMBs commercially available in the market. (1) It is technically unable to form a perfect contact between the solid-state electrolyte and the electrode when fabricating solid-state batteries, which significantly increases the impedance at the electrode/electrolyte interface. (2) Not all the solid electrolytes have a good compatibility with electrodes, thus requiring an additional process to alter the surface nature of solid electrolytes. (3) Relatively slow kinetics of Li-ion transport through the solid-state electrolyte (in comparison with a liquid electrolyte) inhibits the practical use of Li metal anodes in the applications (e.g., EV) requiring fast charging and high power density [27][28]. (4) The manufacturability and material cost of solid-state batteries are not cost-efficient so far, in comparison with that of an liquid electrolyte system [29][30]. In this regard, it would be reasonable to take into account other systems using organic liquid electrolytes and understand what would happen if Li metal is employed in liquid electrolyte-based LIBs.
Metallic Li with “host-less” nature can be infinitely changing during Li plating/stripping process. This leads to a breakdown/restoration of an unstable and non-homogenous solid electrolyte interphase (SEI) layer over the surface of Li metal electrode [11][31][32][33][34]. During Li plating (or termed ‘deposition’), Li + flux is significantly intensified through the cracks of the SEI layer, giving rise to the formation of sharp Li dendrites [31][35][36][37]. The physical features of the formed Li dendrites could lead to the puncture of a polyolefin separator, which dramatically increases the risk of a battery short-circuit. Apart from the formation of Li dendrites, electrochemically dead and isolated Li pillars detached from the Li metal anode are floating inside the cell, which reduces the actual mass of electrochemically active Li ( Figure 1 a) [11]. All of these unwanted phenomena become even more serious when LMBs are operating under abuse conditions, i.e., high/low temperature, high current density, and overcharging. Especially, these topics are of importance since they can directly affect the safety of our lives [38][39][40][41].
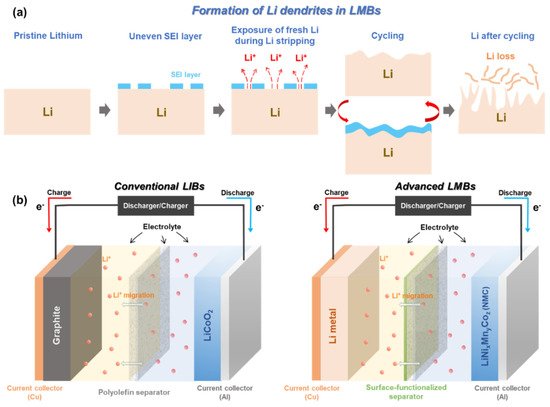
A number of strategies have been proposed to make LMB technology feasible and safer, such as stabilizing the Li electrode surface via interface modification [42][43][44][45][46][47], modifying the chemical constituents of electrolytes [48][49][50][51][52][53][54][55][56][57][58], adding a flame-retardant-based separator [59][60][61][62], and functionalizing the surface of polymer separators [23][63][64][65][66][67]. Encapsulating the Li metal with a protective layer through vapor deposition methods (e.g., physical vapor deposition) has shown a stabilized surface reactivity against atmospheric environments (air and humidity) and has presented optimistic electrochemical results in terms of cycle performance and Coulombic efficiency [43][44][45][46]. Nevertheless, the approach to modify the surface of Li metal via sophisticated techniques is technically hard to achieve both reproducibility and scalability. As to the electrolyte modification, it has somewhat shown impressive effects on suppressing the growth of Li dendrites, enhancing the Coulombic efficiency, and establishing the robust SEI layer [49][50][51]. Moreover, this approach is highly compatible with the conventional battery manufacturing process. Due to these benefits, it is considered the most feasible strategy to resolve the intrinsic problems of Li metal anodes. However, it itself cannot be a fundamental solution to prevent the battery short-circuit caused by Li dendritic growth. In addition, unavoidable side reactions including a parasitic reaction between a Li anode and liquid electrolyte seriously deteriorate the Coulombic efficiency and capacity retention of LMBs [68].
2. Conventional Polymer Separators for LIBs
The porosity, pore size, and thickness are crucial features determining the properties of separators and the overall performances of cells. In general, the pore size and porosity of typical separators is around 1 µm and 40%, respectively [69]. In order to use the separator for practical LMBs, the pore size is required to be small enough (e.g., sub-micrometer) to physically block the penetration of growing Li dendrites, and the porosity should be comparable to typical separators in order to facilitate efficient Li + transfer across the separator. In case of separator thickness, commercially available Celgard’s polymer separators have a thickness of around 25 µm [69]. If the total thickness of the separator gets thicker, it increases the overall resistance of the cell and reduces the loading amount of active materials in the cell. On the contrary, if the total thickness of the separator becomes thinner, it increases the risk of separator puncture by growing Li dendrites.
The battery separator should get wet as soon as it contacts liquid electrolytes. It is reported that the electrolyte wettability of conventional separators is highly associated with battery cycle retention and capacity [56][69][70]. If the separator has a poor electrolyte wettability, it would cause a non-uniform Li-ion transport across the separator. This would result in uneven Li deposition over the electrode, consequently leading to a short circuit of LMBs [71]. To facilitate the homogeneous Li deposition/dissolution in LMBs, it is of paramount importance to improve the electrolyte wettability of separators. The wetting behavior between a separator and a liquid electrolyte is typically studied by using the contact angle measurement [72]. If the separator has a good affinity with liquid electrolytes, the angle between the separator surface and the curvature of an electrolyte droplet would be small. On the other hand, if the separator has a bad affinity with liquid electrolytes, it would have a large contact angle. With these measurements, the electrolyte wetting behavior of separators can be directly determined.
For battery assembly, separators need to meet the following requirements: (1) high mechanical strength to endure the tension and pressure during the battery assembly, (2) excellent insulating property in order not to pass electrons across the separator, and (3) decent electrochemical/chemical stability to use the separator continuously for more than 1000 cycles without degradation [69][73][74]. Especially in the LMB system, the separator should be mechanically strong enough in the liquid cell to suppress the piercing of Li dendrites and the expansion of high-capacity electrodes such as Si and Li and to protect the entire cell from the external stress caused by physical shock and pressure. To evaluate these mechanical properties of separators, mechanical abuse tests (such as nail penetration, puncture, flat crush, and edge crush) and interface-bonding analyses are widely implemented across the industries [75][76][77][78][79][80].
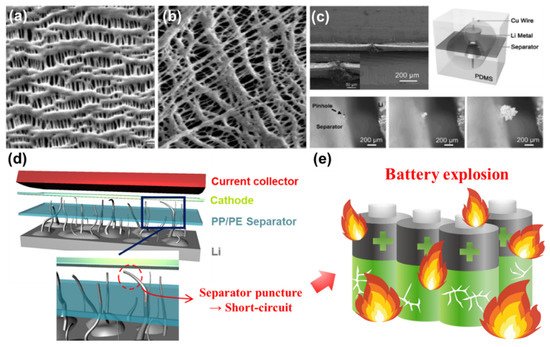
Polypropylene (PP) and polyethylene (PE) are two of the representative polyolefins typically used for fabricating conventional separators [70][73]. The separator fabricated by either wet process or dry process has a number of pores throughout the entire area ( Figure 2 a,b) [69]. Each has good mechanical strength and chemical stability against polar solvents. However, these separators are vulnerable to high temperature and have a relatively poor electrolyte wettability highly associated with the overall resistance in the cell, limiting the practical use for applications (such as EVs) requiring high current levels [69][74]. One of the other serious challenges when a Li metal anode is employed in rechargeable batteries is the separator puncture caused by the penetration of growing Li dendrites. This phenomenon was visually revealed by the transparent cell tests shown in Figure 2 c [67][81][82]. Note that extremely high current flows through the path where Li dendrites penetrate the separator, thus resulting in an exothermic reaction inside the cell followed by an explosion (or catching fire) of LMBs ( Figure 2 d,e) [83]. Typically, the average pore size (e.g., around 1 µm) of polyolefin separators is too large to prevent Li dendrites from piercing the separator. It is reported that the separator with a pore size less than 5 nm has a substantial effect on resisting Li dendrite penetration and redistributing Li + uniformly across the separator [84][85][86]. In order to meet the technical requirements described above and address the potential issues of LMBs, it is of importance to find the optimal condition for fabricating surface-functionalized separators that could maximize the stability and performance of LMBs.
3. Approaches to Modify the Surface of Conventional Polymer Separators for LMBs
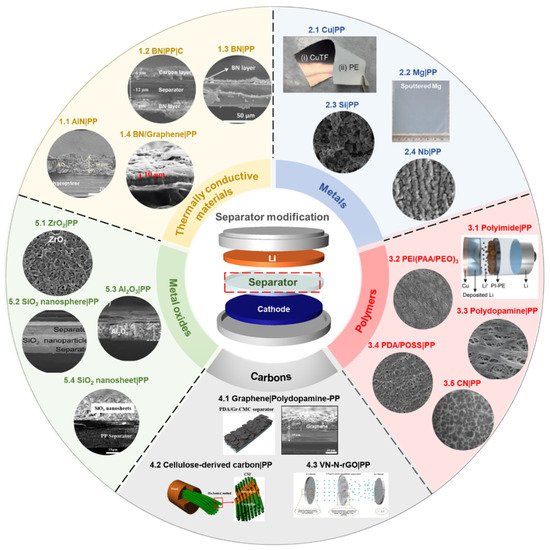
Among the important components of batteries, the battery separator has received relatively less attention than other parts in order to solve the systemic challenges of the Li anode system. The topic of modifying the surface of separators with functional materials has shown promising results in terms of cycle stability and Coulombic efficiency [63][67]. In addition, it is an efficient and straightforward strategy to control the morphology and growing orientation of Li dendrite. Typically, the coating materials to functionalize the surface of polymer separators can be classified into five or six types: (a) thermally conductive materials [63][64][87][88][89][90][91][92], (b) metals [93][94][95][96], (c) polymers [97][98][99][100][101][102], (d) carbons [66][103][104][105], (e) metal oxides [67][81][106][107][108][109][110][111][112][113], and (f) others [114][115][116][117][118] ( Figure 3 ). For electrochemical evaluation, each separator coating material is laminated onto one side or both sides of the polymer separator, using the tape-casting method, physical vapor deposition, etc. The information of separator-coating materials, separators, fabrication techniques, and electrochemical results is listed in the Table 1 .
| Separator-Coating Materials | Separator & Fabrication Technique | Coulombic Efficiency, Current Density, Tested Cells | Ref. |
|---|---|---|---|
| 1. Thermally conductive materials | |||
| AlN nanopowder | PP, Tape-casting | 92% at 1st and 92% at 100th cycle, 0.5 mA cm−2, Li|Cu | [63] |
| BN nanopowder&carbon | PP, Tape-casting | 90% at 1st and 83.5% at 100th cycle, 0.5 mA cm−2, Li|Cu | [64] |
| BN nanosheets | PP/PE/PP, Spray coating | 88% at 1st and 92% at 100th cycle, 0.5 mA cm−2, Li|Cu | [87] |
| BN nanopowder/Graphene | PP, Tape-casting | 77% at 1st and 75% at 100th cycle, 1 mA cm−2, Li|Cu | [91] |
| 2. Metals | |||
| Cu | PE, Magnetron sputtering | 95.4% at 1st and 95.6% at 300th cycle, 0.4 mA cm−2, Li|Cu | [93] |
| Nb | PP, Magnetron sputtering | 97% at 120th cycle, 0.2 C, Li|LNMC | [94] |
| Mg | PP/PE, Magnetron sputtering | 97% at 1st and 94% at 400th cycle, 0.5 mA cm−2, Li|Cu | [95] |
| Si | PP, Tape-casting | 90% at 1st and 97.6% at 100th cycle, 0.5 mA cm−2, Li|Cu | [96] |
| 3. Polymers | |||
| Polyimide | PE, Electrospinning | 98.5% at 700th cycle, 1 mA cm−2, Li|Cu | [97] |
| Polydopamine | PE, Solution immersion | 97.1% at 1st cycle, 0.17 mA cm−2, Li|LiCoO2 | [98] |
| CTS-PEO-PTEGDMA | PP/PE/PP, Electrospraying | ~60% at 1st and 90% at 120th cycle, 0.5 mA cm−2, Li|Cu | [99] |
| PDA/POSS | PE, Dip coating | 98.6% at 200th cycle, 0.2 C, Li|LiCoO2 | [100] |
| PEI(PAA/PEO)3 | PE, Layer-by-layer (LBL) | 99.1% at 400th cycle, 0.2 C, Li|LiCoO2 | [101] |
| C-N polymer | PP, Pasting | ~72% at 1st and 95% at 450th cycle, 3 mA cm−2, Li|Cu | [102] |
| 4. Carbons | |||
| Graphene|Polydopamine | PP, Tape-casting | ~96.1% at 1st and 86.6% at 200th cycle, 0.5 mA cm−2, Li|Cu | [66] |
| VN-N-rGO | PP, Vacuum filtration | 92% at 1st and 97.1% at 55th cycle, 0.5 mA cm−2, Li|Cu | [104] |
| Functionalized nanocarbon | PP, Tape-casting | 96.07–97.41%, 1 mA cm−2, Li|Li | [105] |
| Cellulose-derived carbon | PP, Tape-casting | 36.7% at 1st and 77.1% at 120th cycle, 1 mA cm−2, Li|Cu | [119] |
| 5. Metal oxides | |||
| SiO2 nanosheet | PP, Tape-casting | 81.5% at 1st and 64% at 200th cycle, 1 mA cm−2, Li|Cu | [67] |
| SiO2 nanoparticles | PE, Tape-casting | - | [81] |
| ZrO2 | PE, Self-assembly | 97.8% at 400th cycle, 0.5 C, Li|LiCoO2 | [109] |
| Al2O3 particles | PE, Tape-casting | nearly 100% at 100th cycle, 0.2 C, Li|LiCoO2 | [110] |
| Al2O3 | PVDF-HFP, ALD | nearly 100% at 100th cycle, 0.2 C, Li|LiFePO4 | [111] |
| 6. Others | |||
| Li6.75La3Zr1.75Ta0.25O12 | PP, Tape-casting | 99.5% after 1000 cycles, 0.2 C, Li|LiFePO4 | [114] |
| Al-doped Li6.75La3Zr1.75Ta0.25O12 | PP, Vacuum filtration | 98% after 450 cycles, 0.5 mA cm−2, Li|Cu | [115] |
| Li6.4La3Zr1.4Ta0.6O12 | PP, Tape-casting | 97.5% after 300 cycles, 1 mA cm−2, Li|Cu | [116] |
However, the growth of dendritic Li is still unavoidable, even though the carbon layer on the separator can effectively suppress or change the feature of Li dendrites. In this regard, it would be eventually necessary to do broader research on either resolving the fundamental issue of Li dendrite growth itself or perfectly confining Li dendrites within the anodic side, using a hybrid composition of carbon and other functional materials.
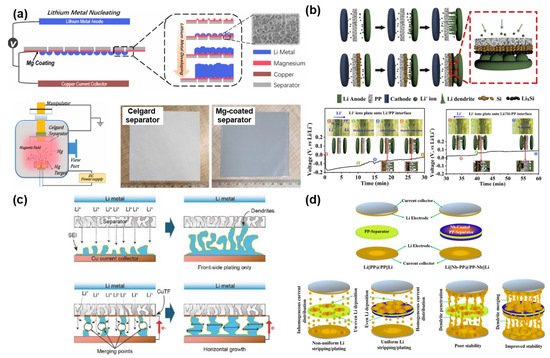
Unlike the previous research studies, there have been different attempts to use metals as functional layers for stabilizing Li anodes. Song et al. suggested a new strategy to control Li dendrite growth by forming a metal (e.g., Mg) layer on one side of the polyolefin separator via sputtering ( Figure 7 a) [95]. The Mg layer with a lithiophilic nature efficiently reduces the Gibbs free energy (ΔG) for Li plating, thus leading to a uniform and dense Li deposition over the counter electrode. The electrochemical results directly corroborated that the lithiophilic Mg layer is able to improve the polarization and cycle stability of the cells having a Li metal anode. Huang et al. demonstrated a “dendrite-eating” separator by coating a Si layer over the PP separator ( Figure 7 b) [96]. The main role of the Si layer is to stabilize Li deposition and reduce the loss of available Li during the repetitive electrochemical reaction. According to the electrochemical data, the symmetrical cell with a Si-coated separator has a smaller polarization and a better cycle stability than the symmetrical cell with a pristine PP separator. This directly indicates the influence of the Si layer on improving the electrochemical performances of LMBs. Transparent symmetric batteries with and without a Si-coated separator were tested to monitor the morphological change of growing Li dendrites as a function of Li plating time. The cell with a Si-coated separator survived longer than the cell with a PP separator. It is because the Si coating not only plays a role in restraining the dendrite formation by alloying with Li dendrites but also distributes Li + flux homogeneously across the separator. Zhang et al. proposed depositing an ultrathin Cu film on a PE separator for improving the electrochemical stability ( Figure 7 c) [93]. The ultrathin Cu film can change the growth orientation of Li dendrites from the vertical direction to the horizontal direction as well as diminish the local current density by exposing more surface area of the Li layer. Due to these beneficial functions of the ultrathin Cu layer, the cycle performance and Coulombic efficiency of Li|Cu cells were dramatically ameliorated in comparison with the Li|Cu cell with a pristine PE separator. Murugan et al. reported a binder-free Nb-coated PP separator for LMBs ( Figure 4 d) [94]. The Nb layer alters the surface nature of the PP separator from hydrophobicity to hydrophilicity, contributing to an improved electrolyte wettability. In addition, the formed metal layer not only enhances the mechanical strength of the conventional PP separator but also serves as an additional conductive path inducing the merge of Li dendrites from both sides of the anode and Nb layer. Moreover, the improved contact between the Nb layer and Li anode and the homogeneous current flow efficiently reduces the interfacial resistance between the anode and electrolyte. All these advantageous features greatly mitigate the risk of a short circuit and improve the cycle life with small polarization in LMBs.
Polymers are extensively used as binding materials, separators, and electrolytes in rechargeable batteries due to the high mechanical stability, the excellent chemical resistance, and the good adhesion properties [120]. In addition to these purposes, polymers are also adopted as coating materials for separator modification.
4. Conclusions and Outlooks
Typical polyolefin separators are not physically robust enough to restrain the propagation of growing Li/Na dendrites, which allows the unavoidable penetration of dendritic Li through the separator. In addition, polyolefin separators are not thermally stable to maintain its structural integrity at high temperatures and have relatively poor electrolyte wettability. These material limits hinder the practical use of polymer separators for high-performance batteries with Li metal anode. The surface modification of polymer separators with functional materials can play an important role in homogenizing Li + flux, preventing a physical penetration of alkaline metal dendrite, and strengthening a thermal/mechanical stability of separators. This paper reviews the characteristics and limits of existing battery separators and summarizes the overall separator-coating materials that are effective in controlling the growth of dendritic Li and improving the efficiency of reversible Li + transport. Surface-functionalized separators designed for LMBs can be classified into five or six categories according to the types of separator coating materials: (a) thermally conductive materials, (b) metal oxides, (c) carbons, (d) polymers, (e) metals, and (f) others (including solid-state electrolytes). Each type of material has different material properties, directly leading to different electrochemical results when applied to the surface of polymer separators for LMBs.
To date, there has been meaningful progress in fabricating functional separators for next-generation batteries including Li-S batteries and Li/Na metal batteries. With the adoption of the customized separators, the battery’s electrochemical/thermal stability has been remarkably improved in comparison to the existing battery technologies. These technologies are currently used by global battery industries (e.g., LG Chem., Samsung SDI, SK Innovation, etc.). Unfortunately, further improvements are still required to use the surface-modified separator over the long term and reduce the side reactions that mostly happen inside the Li metal cell. For example, it is no longer possible to homogenize Li + flux and block the Li dendrite penetration once the coating layer is fully covered by metallic Li during the electrochemical test. It is because the Li-deposited layer itself can serve as another Li anode inside the cell.
Considering the potential problems, there are a few parts where researchers and engineers can be devoted to upgrading the previous approaches. For instance, constructing a hybrid film comprising more than two types of separator-coating materials (described in Figure 3 ) on the polymer separator might be a feasible strategy to have multiple synergistic effects on improving the reversibility and stability of LMBs. The combination of a gel electrolyte with a surface-functionalized separator would be another approach to address the interface issues such as a non-uniform contact between a coating layer and an electrode. Alongside these suggested ideas, there are many niche markets where researchers can contribute to the further improvement of battery separators for practical LMBs. In addition to these conceptual approaches, the cost reduction of manufacturing functional separators is another important factor to be considered for successful commercialization. Since most of the previous studies were mainly focused on fabricating the surface-functionalized separator on a laboratory scale (using either tape-casting or sputtering), it would significantly increase the manufacturing cost of functional separators compatible with the battery assembly process.
In this regard, there are still many hurdles for researchers to go through. The topic of surface-functionalized separators needs more relevant and meaningful research to overcome the inherent weaknesses of polymer separators and open a new route to customize the properties of polymer separators.
This entry is adapted from the peer-reviewed paper 10.3390/nano11092275
References
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337.
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657.
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176.
- Brandt, K. Historical development of secondary lithium batteries. Solid State Ion. 1994, 69, 173–183.
- Scrosati, B. History of lithium batteries. J. Solid State Electr. 2011, 15, 1623–1630.
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief history of early lithium-battery development. Materials 2020, 13, 1884.
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800.
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288.
- Levin, K.; Fransen, T.; Schumer, C.; Davis, C. What Does” Net-Zero Emissions” Mean? 8 Common Questions, Answered. World Resources Institute. Available online: https://www.wri.org/insights/net-zero-ghg-emissions-questions-answered (accessed on 17 September 2019).
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing lithium metal batteries. Joule 2018, 2, 833–845.
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206.
- Whittingham, M.S. Electrical energy storage and intercalation chemistry. Science 1976, 192, 1126–1127.
- Winter, M.; Barnett, B.; Xu, K. Before Li ion batteries. Chem. Rev. 2018, 118, 11433–11456.
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O 2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29.
- Chen, H.; Xiao, Y.; Chen, C.; Yang, J.; Gao, C.; Chen, Y.; Wu, J.; Shen, Y.; Zhang, W.; Li, S. Conductive MOF-modified separator for mitigating the shuttle effect of lithium–sulfur battery through a filtration method. ACS Appl. Mater. Interfaces 2019, 11, 11459–11465.
- Zhao, Y.; Liu, M.; Lv, W.; He, Y.-B.; Wang, C.; Yun, Q.; Li, B.; Kang, F.; Yang, Q.-H. Dense coating of Li4Ti5O12 and graphene mixture on the separator to produce long cycle life of lithium-sulfur battery. Nano Energy 2016, 30, 1–8.
- Paolella, A.; Demers, H.; Chevallier, P.; Gagnon, C.; Girard, G.; Delaporte, N.; Zhu, W.; Vijh, A.; Guerfi, A.; Zaghib, K. A platinum nanolayer on lithium metal as an interfacial barrier to shuttle effect in Li-S batteries. J. Power Sources 2019, 427, 201–206.
- Qian, X.; Jin, L.; Zhao, D.; Yang, X.; Wang, S.; Shen, X.; Rao, D.; Yao, S.; Zhou, Y.; Xi, X. Ketjen black-MnO composite coated separator for high performance rechargeable lithium-sulfur battery. Electrochim. Acta 2016, 192, 346–356.
- Paolella, A.; Laul, D.; Timoshevskii, V.; Zhu, W.; Marras, S.; Bertoni, G.; Wahba, A.S.; Girard, G.; Gagnon, C.; Rodrigue, L. The role of metal disulfide interlayer in Li–S batteries. J. Phys. Chem. C 2018, 122, 1014–1023.
- Xu, Q.; Zhang, K.; Qian, J.; Guo, Y.; Song, X.; Pan, H.; Wang, D.; Li, X. Boosting lithium–sulfur battery performance by integrating a redox-active covalent organic framework in the separator. ACS Appl. Energy Mater. 2019, 2, 5793–5798.
- Kim, J.H.; Fu, K.; Choi, J.; Sun, S.; Kim, J.; Hu, L.; Paik, U. Hydroxylated carbon nanotube enhanced sulfur cathodes for improved electrochemical performance of lithium–sulfur batteries. Chem. Commun. 2015, 51, 13682–13685.
- Kim, J.H.; Fu, K.; Choi, J.; Kil, K.; Kim, J.; Han, X.; Hu, L.; Paik, U. Encapsulation of S/SWNT with PANI web for enhanced rate and cycle performance in lithium sulfur batteries. Sci. Rep. 2015, 5, 1–6.
- Kim, P.J.H.; Kim, K.; Pol, V.G. Towards highly stable lithium sulfur batteries: Surface functionalization of carbon nanotube scaffolds. Carbon 2018, 131, 175–183.
- Kim, J.H.; Choi, J.; Seo, J.; Kwon, J.; Paik, U. Two-dimensional Nafion nanoweb anion-shield for improved electrochemical performances of lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 11203–11206.
- Kim, K.; Kim, P.J.; Youngblood, J.P.; Pol, V.G. Surface Functionalization of Carbon Architecture with Nano-MnO2 for Effective Polysulfide Confinement in Lithium–Sulfur Batteries. ChemsusChem. 2018, 11, 2375–2381.
- Lee, Y.-G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.-S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 2020, 5, 299–308.
- Albertus, P.; Anandan, V.; Ban, C.; Balsara, N.; Belharouak, I.; Buettner-Garrett, J.; Chen, Z.; Daniel, C.; Doeff, M.; Dudney, N.J. Challenges for and pathways toward Li-metal-based all-solid-state batteries. ACS Energy Lett. 2021, 6, 1399–1404.
- Janek, J.; Zeier, W.G. A solid future for battery development. Nature Energy 2016, 1, 1–4.
- Kerman, K.; Luntz, A.; Viswanathan, V.; Chiang, Y.-M.; Chen, Z. Practical challenges hindering the development of solid state Li ion batteries. J. Electrochem. Soc. 2017, 164, A1731.
- Hu, Y.-S. Batteries: Getting solid. Nat. Energy 2016, 1, 1–2.
- Liu, D.-H.; Bai, Z.; Li, M.; Yu, A.; Luo, D.; Liu, W.; Yang, L.; Lu, J.; Amine, K.; Chen, Z. Developing high safety Li-metal anodes for future high-energy Li-metal batteries: Strategies and perspectives. Chem. Soc. Rev. 2020, 49, 5407–5445.
- Wang, M.; Sakamoto, J. Correlating the interface resistance and surface adhesion of the Li metal-solid electrolyte interface. J. Power Sources 2018, 377, 7–11.
- Zhang, S.; Xu, K.; Jow, T. EIS study on the formation of solid electrolyte interface in Li-ion battery. Electrochim. Acta 2006, 51, 1636–1640.
- Liu, S.; Zhang, Q.; Wang, X.; Xu, M.; Li, W.; Lucht, B.L. LiFSI and LiDFBOP dual-salt electrolyte reinforces the solid electrolyte interphase on a lithium metal anode. ACS Appl. Mater. Interfaces 2020, 12, 33719–33728.
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.; Shao, Y.; Engelhard, M.H.; Nie, Z.; Xiao, J. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456.
- Harry, K.J.; Hallinan, D.T.; Parkinson, D.Y.; MacDowell, A.A.; Balsara, N.P. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 2014, 13, 69–73.
- Liu, Y.; Lin, D.; Liang, Z.; Zhao, J.; Yan, K.; Cui, Y. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 2016, 7, 1–9.
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Mater. 2020, 24, 85–112.
- Wu, H.; Xie, Z.; Wang, Y.; Zhang, P.; Sun, L.; Lu, C.; Ma, Z. A constitutive model coupling irradiation with two-phase lithiation for lithium-ion battery electrodes. Philos. Mag. 2019, 99, 992–1013.
- Duan, X.; Jiang, W.; Zou, Y.; Lei, W.; Ma, Z. A coupled electrochemical–thermal–mechanical model for spiral-wound Li-ion batteries. J. Mater. Sci 2018, 53, 10987–11001.
- Ma, Z.; Wu, H.; Wang, Y.; Pan, Y.; Lu, C. An electrochemical-irradiated plasticity model for metallic electrodes in lithium-ion batteries. Int. J. Plast. 2017, 88, 188–203.
- Kozen, A.C.; Lin, C.-F.; Pearse, A.J.; Schroeder, M.A.; Han, X.; Hu, L.; Lee, S.-B.; Rubloff, G.W.; Noked, M. Next-generation lithium metal anode engineering via atomic layer deposition. ACS Nano 2015, 9, 5884–5892.
- Kazyak, E.; Wood, K.N.; Dasgupta, N.P. Improved cycle life and stability of lithium metal anodes through ultrathin atomic layer deposition surface treatments. Chem. Mater. 2015, 27, 6457–6462.
- Kozen, A.C.; Lin, C.-F.; Zhao, O.; Lee, S.B.; Rubloff, G.W.; Noked, M. Stabilization of lithium metal anodes by hybrid artificial solid electrolyte interphase. Chem. Mater. 2017, 29, 6298–6307.
- Kim, J.Y.; Kim, A.-Y.; Liu, G.; Woo, J.-Y.; Kim, H.; Lee, J.K. Li4SiO4-based artificial passivation thin film for improving interfacial stability of Li metal anodes. ACS Appl. Mater. Interfaces 2018, 10, 8692–8701.
- Chen, L.; Connell, J.G.; Nie, A.; Huang, Z.; Zavadil, K.R.; Klavetter, K.C.; Yuan, Y.; Sharifi-Asl, S.; Shahbazian-Yassar, R.; Libera, J.A. Lithium metal protected by atomic layer deposition metal oxide for high performance anodes. J. Mater. Chem. A 2017, 5, 12297–12309.
- Wang, L.; Zhang, L.; Wang, Q.; Li, W.; Wu, B.; Jia, W.; Wang, Y.; Li, J.; Li, H. Long lifespan lithium metal anodes enabled by Al2O3 sputter coating. Energy Storage Mater. 2018, 10, 16–23.
- Ren, X.; Zhang, Y.; Engelhard, M.H.; Li, Q.; Zhang, J.-G.; Xu, W. Guided lithium metal deposition and improved lithium coulombic efficiency through synergistic effects of LiAsF6 and cyclic carbonate additives. ACS Energy Lett. 2017, 3, 14–19.
- Yang, H.; Li, J.; Sun, Z.; Fang, R.; Wang, D.-W.; He, K.; Cheng, H.-M.; Li, F. Reliable liquid electrolytes for lithium metal batteries. Energy Storage Mater. 2020, 30, 113–129.
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.-P.; Phan, T.N.; Bertin, D.; Gigmes, D.; Devaux, D. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457.
- Han, F.; Yue, J.; Zhu, X.; Wang, C. Suppressing Li dendrite formation in Li2S-P2S5 solid electrolyte by LiI incorporation. Adv. Energy Mater. 2018, 8, 1703644.
- Zheng, J.; Engelhard, M.H.; Mei, D.; Jiao, S.; Polzin, B.J.; Zhang, J.-G.; Xu, W. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2017, 2, 1–8.
- Wang, G.; Xiong, X.; Xie, D.; Fu, X.; Ma, X.; Li, Y.; Liu, Y.; Lin, Z.; Yang, C.; Liu, M. Suppressing dendrite growth by a functional electrolyte additive for robust Li metal anodes. Energy Storage Mater. 2019, 23, 701–706.
- Zhang, S.; Yang, G.; Liu, Z.; Li, X.; Wang, X.; Chen, R.; Wu, F.; Wang, Z.; Chen, L. Competitive Solvation Enhanced Stability of Lithium Metal Anode in Dual-Salt Electrolyte. Nano Lett. 2021, 21, 3310–3317.
- Wang, D.; Liu, H.; Li, M.; Xia, D.; Holoubek, J.; Deng, Z.; Yu, M.; Tian, J.; Shan, Z.; Ong, S.P. A long-lasting dual-function electrolyte additive for stable lithium metal batteries. Nano Energy 2020, 75, 104889.
- Francis, C.F.; Kyratzis, I.L.; Best, A.S. Lithium-Ion Battery Separators for Ionic-Liquid Electrolytes: A Review. Adv. Mater. 2020, 32, 1904205.
- Hung, L.; Chen, C. Materials science and engineering: R: Reports. Mater. Sci. Eng. 2002, 39, 143–222.
- Nie, M.; Lucht, B.L. Role of lithium salt on solid electrolyte interface (SEI) formation and structure in lithium ion batteries. J. Electrochem. Soc. 2014, 161, A1001.
- Liu, K.; Liu, W.; Qiu, Y.; Kong, B.; Sun, Y.; Chen, Z.; Zhuo, D.; Lin, D.; Cui, Y. Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 2017, 3, e1601978.
- Delaporte, N.; Perea, A.; Paolella, A.; Dubé, J.; Vigeant, M.-J.; Demers, H.; Clément, D.; Zhu, W.; Gariépy, V.; Zaghib, K. Alumina-flame retardant separators toward safe high voltage Li-Ion batteries. J. Power Sources 2021, 506, 230189.
- Luo, X.; Lu, X.; Chen, X.; Chen, Y.; Song, C.; Yu, C.; Wang, N.; Su, D.; Wang, C.; Gao, X. A robust flame retardant fluorinated polyimide nanofiber separator for high-temperature lithium–sulfur batteries. J. Mater. Chem. A 2020, 8, 14788–14798.
- Wu, N.; Wang, J.; Liao, C.; Han, L.; Song, L.; Hu, Y.; Mu, X.; Kan, Y. A flame retardant separator modified by MOFs-derived hybrid for safe and efficient Li-S batteries. J. Energy Chem. 2022, 64, 372–384.
- Kim, P.J.H.; Pol, V.G. Surface functionalization of a conventional polypropylene separator with an aluminum nitride layer toward ultrastable and hsigh-rate lithium metal anodes. ACS Appl. Mater. Interfaces 2019, 11, 3917–3924.
- Kim, P.J.H.; Seo, J.; Fu, K.; Choi, J.; Liu, Z.; Kwon, J.; Hu, L.; Paik, U. Synergistic protective effect of a BN-carbon separator for highly stable lithium sulfur batteries. NPG Asia Mater. 2017, 9, e375.
- Kim, P.J.; Fontecha, H.D.; Kim, K.; Pol, V.G. Toward High-Performance Lithium-Sulfur Batteries: Upcycling of LDPE Plastic into Sulfonated Carbon Scaffold via Microwave-Promoted 66. ACS Appl. Mater. Interfaces 2018, 10, 14827–14834.
- Kim, P.J.; Pol, V.G. High performance lithium metal batteries enabled by surface tailoring of polypropylene separator with a polydopamine/graphene layer. Adv. Energy Mater. 2018, 8, 1802665.
- Kim, P.J.; Kim, K.; Pol, V.G. Uniform metal-ion flux through interface-modified membrane for highly stable metal batteries. Electrochim. Acta 2018, 283, 517–527.
- Xie, J.; Wang, J.; Lee, H.R.; Yan, K.; Li, Y.; Shi, F.; Huang, W.; Pei, A.; Chen, G.; Subbaraman, R. Engineering stable interfaces for three-dimensional lithium metal anodes. Sci. Adv. 2018, 4, eaat5168.
- Arora, P.; Zhang, Z.J. Battery separators. Chem. Rev. 2004, 104, 4419–4462.
- Huang, X. Separator technologies for lithium-ion batteries. J. Solid State Electr. 2011, 15, 649–662.
- Jeon, D.H. Wettability in electrodes and its impact on the performance of lithium-ion batteries. Energy Storage Mater. 2019, 18, 139–147.
- Zhang, J.; Yue, L.; Kong, Q.; Liu, Z.; Zhou, X.; Zhang, C.; Xu, Q.; Zhang, B.; Ding, G.; Qin, B. Sustainable, heat-resistant and flame-retardant cellulose-based composite separator for high-performance lithium ion battery. Sci. Rep. 2014, 4, 1–8.
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energ. Environ. Sci. 2014, 7, 3857–3886.
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364.
- Sahraei, E.; Campbell, J.; Wierzbicki, T. Modeling and short circuit detection of 18650 Li-ion cells under mechanical abuse conditions. J. Power Sources 2012, 220, 360–372.
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100.
- Wang, Y.; Pu, Y.; Ma, Z.; Pan, Y.; Sun, C.Q. Interfacial adhesion energy of lithium-ion battery electrodes. Extrem. Mech. Lett. 2016, 9, 226–236.
- Wang, C.; Ma, Z.; Wang, Y.; Lu, C. Failure prediction of high-capacity electrode materials in lithium-ion batteries. J. Electrochem. Soc. 2016, 163, A1157.
- Ma, Z.; Xie, Z.; Wang, Y.; Zhang, P.; Pan, Y.; Zhou, Y.; Lu, C. Failure modes of hollow core–shell structural active materials during the lithiation–delithiation process. J. Power Sources 2015, 290, 114–122.
- Zhang, P.; Wang, Y.; Lei, W.; Zou, Y.; Jiang, W.; Ma, Z.; Lu, C. Enhancement Effects of Co Doping on Interfacial Properties of Sn Electrode− Collector: A First-Principles Study. ACS Appl. Mater. Interfaces 2019, 11, 24648–24658.
- Liu, K.; Zhuo, D.; Lee, H.W.; Liu, W.; Lin, D.; Lu, Y.; Cui, Y. Extending the life of lithium-based rechargeable batteries by reaction of lithium dendrites with a novel silica nanoparticle sandwiched separator. Adv. Mater. 2017, 29, 1603987.
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820.
- Lisbona, D.; Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf. Environ. Prot. 2011, 89, 434–442.
- Deimede, V.; Elmasides, C. Separators for lithium-ion batteries: A review on the production processes and recent developments. Energy Technol. 2015, 3, 453–468.
- Bai, P.; Guo, J.; Wang, M.; Kushima, A.; Su, L.; Li, J.; Brushett, F.R.; Bazant, M.Z. Interactions between lithium growths and nanoporous ceramic separators. Joule 2018, 2, 2434–2449.
- Yang, J.; Wang, C.-Y.; Wang, C.-C.; Chen, K.-H.; Mou, C.-Y.; Wu, H.-L. Advanced nanoporous separators for stable lithium metal electrodeposition at ultra-high current densities in liquid electrolytes. J. Mater. Chem. A 2020, 8, 5095–5104.
- Luo, W.; Zhou, L.; Fu, K.; Yang, Z.; Wan, J.; Manno, M.; Yao, Y.; Zhu, H.; Yang, B.; Hu, L. A Thermally Conductive Separator for Stable Li Metal Anodes. Nano Lett. 2015, 15, 6149–6154.
- Gao, C.; Hong, B.; Sun, K.; Fan, H.; Zhang, K.; Zhang, Z.; Lai, Y. Self-Suppression of Lithium Dendrite with Aluminum Nitride Nanoflake Additive in 3D Carbon Paper for Lithium Metal Batteries. Energy Technol. 2020, 8, 1901463.
- Liu, Y.; Qiao, Y.; Zhang, Y.; Yang, Z.; Gao, T.; Kirsch, D.; Liu, B.; Song, J.; Yang, B.; Hu, L. 3D printed separator for the thermal management of high-performance Li metal anodes. Energy Storage Mater. 2018, 12, 197–203.
- Shen, B.; Zhang, T.-W.; Yin, Y.-C.; Zhu, Z.-X.; Lu, L.-L.; Ma, C.; Zhou, F.; Yao, H.-B. Chemically exfoliated boron nitride nanosheets form robust interfacial layers for stable solid-state Li metal batteries. Chem. Commun. 2019, 55, 7703–7706.
- Rodriguez, J.R.; Kim, P.J.; Kim, K.; Qi, Z.; Wang, H.; Pol, V.G. Engineered heat dissipation and current distribution boron nitride-graphene layer coated on polypropylene separator for high performance lithium metal battery. J. Colloid Interface Sci. 2021, 583, 362–370.
- Cheng, Q.; Li, A.; Li, N.; Li, S.; Zangiabadi, A.; Huang, W.; Li, A.C.; Jin, T.; Song, Q.; Xu, W. Stabilizing solid electrolyte-anode interface in Li-metal batteries by boron nitride-based nanocomposite coating. Joule 2019, 3, 1510–1522.
- Lee, H.; Ren, X.; Niu, C.; Yu, L.; Engelhard, M.H.; Cho, I.; Ryou, M.H.; Jin, H.S.; Kim, H.T.; Liu, J. Suppressing lithium dendrite growth by metallic coating on a separator. Adv. Funct. Mater. 2017, 27, 1704391.
- Din, M.M.U.; Murugan, R. Metal coated polypropylene separator with enhanced surface wettability for high capacity lithium metal batteries. Sci. Rep. 2019, 9, 1–12.
- Liu, Y.; Xiong, S.; Wang, J.; Jiao, X.; Li, S.; Zhang, C.; Song, Z.; Song, J. Dendrite-free lithium metal anode enabled by separator engineering via uniform loading of lithiophilic nucleation sites. Energy Storage Mater. 2019, 19, 24–30.
- Chen, X.; Zhang, R.; Zhao, R.; Qi, X.; Li, K.; Sun, Q.; Ma, M.; Qie, L.; Huang, Y. A “dendrite-eating” separator for high-areal-capacity lithium-metal batteries. Energy Storage Mater. 2020, 31, 181–186.
- Yin, Y.; Wang, K.; Shen, F.; Han, X. Dendrite-Suppressing Separator with High Thermal Stability Modified by Beaded-Chain-Like Polyimide Coating for a Li Metal Anode. Energy Fuels 2021, 35, 8417–8422.
- Ryou, M.H.; Lee, D.J.; Lee, J.N.; Lee, Y.M.; Park, J.K.; Choi, J.W. Excellent cycle life of lithium-metal anodes in lithium-ion batteries with mussel-inspired polydopamine-coated separators. Adv. Energy Mater. 2012, 2, 645–650.
- Shen, L.; Liu, X.; Dong, J.; Zhang, Y.; Xu, C.; Lai, C.; Zhang, S. Functional lithiophilic polymer modified separator for dendrite-free and pulverization-free lithium metal batteries. J. Energy Chem. 2021, 52, 262–268.
- Wang, Y.; Shi, L.; Zhou, H.; Wang, Z.; Li, R.; Zhu, J.; Qiu, Z.; Zhao, Y.; Zhang, M.; Yuan, S. Polyethylene separators modified by ultrathin hybrid films enhancing lithium ion transport performance and Li-metal anode stability. Electrochim. Acta 2018, 259, 386–394.
- Jin, R.; Fu, L.; Zhou, H.; Wang, Z.; Qiu, Z.; Shi, L.; Zhu, J.; Yuan, S. High Li+ ionic flux separator enhancing cycling stability of lithium metal anode. ACS. Sustain. Chem. Eng. 2018, 6, 2961–2968.
- Yang, Q.; Cui, M.; Hu, J.; Chu, F.; Zheng, Y.; Liu, J.; Li, C. Ultrathin Defective C–N Coating to Enable Nanostructured Li Plating for Li Metal Batteries. ACS Nano 2020, 14, 1866–1878.
- Kim, J.Y.; Shin, D.O.; Kim, K.M.; Oh, J.; Kim, J.; Kang, S.H.; Lee, M.J.; Lee, Y.-G. Graphene oxide induced surface modification for functional separators in lithium secondary batteries. Sci. Rep. 2019, 9, 1–7.
- Zhang, X.; Chen, Y.; Yu, B.; Wang, B.; Wang, X.; Zhang, W.; Yang, D.; He, J. Lithiophilic 3D [email protected] N-rGO as a Multifunctional Interlayer for Dendrite-Free and Ultrastable Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 20125–20136.
- Liu, Y.; Liu, Q.; Xin, L.; Liu, Y.; Yang, F.; Stach, E.A.; Xie, J. Making Li-metal electrodes rechargeable by controlling the dendrite growth direction. Nat. Energy 2017, 2, 1–10.
- Na, W.; Koh, K.H.; Lee, A.S.; Cho, S.; Ok, B.; Hwang, S.-W.; Lee, J.H.; Koo, C.M. Binder-less chemical grafting of SiO2 nanoparticles onto polyethylene separators for lithium-ion batteries. J. Membr. Sci. 2019, 573, 621–627.
- Kang, S.M.; Ryou, M.-H.; Choi, J.W.; Lee, H. Mussel-and diatom-inspired silica coating on separators yields improved power and safety in Li-ion batteries. Chem. Mater. 2012, 24, 3481–3485.
- Zhu, M.; Wang, Q.; Zhou, H.; Qi, L. Binder-Free TiO2-Coated Polypropylene Separators for Advanced Lithium-Ion Batteries. Energy Technol. 2020, 8, 2000228.
- Chi, M.; Shi, L.; Wang, Z.; Zhu, J.; Mao, X.; Zhao, Y.; Zhang, M.; Sun, L.; Yuan, S. Excellent rate capability and cycle life of Li metal batteries with ZrO2/POSS multilayer-assembled PE separators. Nano Energy 2016, 28, 1–11.
- Wang, Q.; Yang, J.; Wang, Z.; Shi, L.; Zhao, Y.; Yuan, S. Dual-Scale Al2O3 Particles Coating for High-Performance Separator and Lithium Metal Anode. Energy Technol. 2020, 8, 1901429.
- Wang, W.; Yuan, Y.; Wang, J.; Zhang, Y.; Liao, C.; Mu, X.; Sheng, H.; Kan, Y.; Song, L.; Hu, Y. Enhanced electrochemical and safety performance of lithium metal batteries enabled by the atom layer deposition on PVDF-HFP separator. ACS Appl. Energy Mater. 2019, 2, 4167–4174.
- Jeon, H.; Jin, S.Y.; Park, W.H.; Lee, H.; Kim, H.-T.; Ryou, M.-H.; Lee, Y.M. Plasma-assisted water-based Al2O3 ceramic coating for polyethylene-based microporous separators for lithium metal secondary batteries. Electrochim. Acta 2016, 212, 649–656.
- Jung, B.; Lee, B.; Jeong, Y.-C.; Lee, J.; Yang, S.R.; Kim, H.; Park, M. Thermally stable non-aqueous ceramic-coated separators with enhanced nail penetration performance. J. Power Sources 2019, 427, 271–282.
- Mao, Y.; Sun, W.; Qiao, Y.; Liu, X.; Xu, C.; Fang, L.; Hou, W.; Wang, Z.; Sun, K. A high strength hybrid separator with fast ionic conductor for dendrite-free lithium metal batteries. Chem. Eng. J. 2021, 416, 129119.
- Zhao, C.-Z.; Chen, P.-Y.; Zhang, R.; Chen, X.; Li, B.-Q.; Zhang, X.-Q.; Cheng, X.-B.; Zhang, Q. An ion redistributor for dendrite-free lithium metal anodes. Sci. Adv. 2018, 4, eaat3446.
- Huo, H.; Li, X.; Chen, Y.; Liang, J.; Deng, S.; Gao, X.; Doyle-Davis, K.; Li, R.; Guo, X.; Shen, Y. Bifunctional composite separator with a solid-state-battery strategy for dendrite-free lithium metal batteries. Energy Storage Mater. 2020, 29, 361–366.
- Yan, J.; Liu, F.; Hu, Z.; Gao, J.; Zhou, W.; Huo, H.; Zhou, J.; Li, L. Realizing dendrite-free lithium deposition with a composite separator. Nano Lett. 2020, 20, 3798–3807.
- Han, D.-H.; Zhang, M.; Lu, P.-X.; Wan, Y.-L.; Chen, Q.-L.; Niu, H.-Y.; Yu, Z.-W. A multifunctional separator with Mg (OH) 2 nanoflake coatings for safe lithium-metal batteries. J. Energy Chem. 2021, 52, 75–83.
- Kim, P.J.; Kim, K.; Pol, V.G. A comparative study of cellulose derived structured carbons on the electrochemical behavior of lithium metal-based batteries. Energy Storage Mater. 2019, 19, 179–185.
- Lopez, J.; Mackanic, D.G.; Cui, Y.; Bao, Z. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 2019, 4, 312–330.
