Volumetric muscle loss (VML) is defined as a condition in which a large volume of skeletal muscle is lost due to physical insult. VML often results in a heightened immune response, resulting in significant long-term functional impairment.
- VML
- immune response
- biomaterials
- reconstructive therapies
1. Introduction
Voluntary muscle or skeletal muscle comprises 40%–50% of the human body, and plays a major role in locomotion, breathing, and posture support [1]. Multiple skeletal muscular diseases adversely affect life and, among these, the most common are muscular dystrophy, [2] cardiomyopathy, [3] myasthenia gravis, [4] poly- and dermatomyositis, and rhabdomyolysis. Interestingly skeletal muscle has an intrinsic ability for regeneration after mild injury. However, volumetric muscle loss [2] that occurs due to surgical or traumatic excision of a portion of skeletal muscle presents an irrevocable condition in humans which leads to sequelae of chronic trauma [5]. VML represents an alarming situation for the U.S. Military Health System, as VML is the main source of disability among service personnel, as well as being important in the civilian population [6][7].
There is a well-known correlation between muscle regeneration and inflammation following acute injury. A mechanistic correlation between muscle inflammation as a result of both innate and adaptive immune dysregulation and regeneration has been recently provided by cellular immunologists, developmental biologists, and muscle pathophysiologists [8]. The in-practice treatment for VML surgery is a follow-up exercise which results in recovery of muscle and its functions. However, the scientific community is struggling with hard to rely on reconstructive therapy for VML. Perhaps most gratifying is that recent approaches have led to new ways to improve inflammatory responses to muscle regeneration both in chronic disease and muscle trauma. Till now, various methodologies, such as activation of immune responses [9], biological scaffolds [10][11], tissue engineering [12], hydrogels [13], and cell transplantation [14], have been utilized to overcome VML.
2. Role of Immune Response in VML
Leukocytes are a non-obtrusive element of skeletal muscle that consists of a small portion of intramuscular neutrophils, eosinophils, CD8 + cytotoxic T cells, and regulatory T (Treg) cells. It is well known that the larger population of intramuscular leukocytes is composed of monocytes or macrophages [15]. These cells are in the connective tissue sheath that surrounds muscle or blood vessels. The tissue-resident macrophages are also located in a dormant state in healthy muscles. In the case of muscle injury or sudden trauma, the dormant macrophages become rapidly activated and play a role in muscle regeneration or an enhanced wound healing response ( Figure 1 ). As compared to other immune cells, the neutrophils and macrophages are the first line defender in muscle regeneration after any insult or trauma [16]. Following injury, the various immune cells are activated at the spot to remove necrotic cells and to release cytokines [17]. Initially, upon activation, the macrophages secrete tumor necrosis factor α (TNFα), interleukin-1β (IL-1β), and interferon-gamma (IFN-γ), which are also known as pro-inflammatory cytokines, to facilitate cell debris removal. These macrophages further recruit other immune cells at the injured site and they later switch to the anti-inflammatory response that releases anti-inflammatory cytokines to suppress the local inflammatory response and enhance muscle growth [18]. Interestingly, during the phagocytosis of damaged muscle, M1 macrophages reduce TNFα levels, C-C motif chemokine ligand 3 (CCL3), and inducible nitric oxide synthase (iNOS), whereas they enhance expression of CD163, TGFβ1, CD206 by switching to the M2 phenotype. These M2 macrophages are alternatively activated and secrete IL-10 [19][20][21]. The pro-inflammatory macrophages boost the motility of myogenic cells while exerting adverse effects on their differentiation. Furthermore, it has been shown that other major players like hepatocyte growth factor (HGF), insulin-like growth factor-1 (IGF-1), fibroblast growth factor-2 (FGF-2), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and Notch signaling in the proliferation of satellite cells plays a significant role in muscle rejuvenation [20][22]. The proliferation in satellite cells results in new dormant satellite cells and myogenic progenitors, which prompt Myf5, MyoD, Mrf4, and myogenin [23][24]. In humans, the affected area contains myogenic progenitors that stimulate macrophages, articulating pro-inflammatory markers like IL-1α, -β, IL-6, TNF-α, and nitric oxide synthase 2 (NOS2) [25][26][27]. However, in vitro analysis reveals that M2 and anti-inflammatory macrophages enhance differentiation of myogenic progenitor [27]. The regenerating muscle contains differentiating myogenic progenitors that are linked with macrophages that protect the anti-inflammatory markers IL4, IL5, IL10, and TGFβ [25][28].
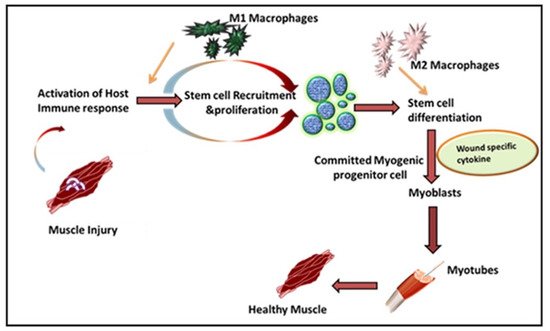
The freshly formed fibers may form an assortment of progressive patterns. The created fibers are forked as newly generated myofibers are fused in an incomplete pattern. Satellite cells also fuse with myofibers under the basal lamina of previously existing fibers [29]. To restore the growth and function of muscles, the mandatory factors are neuro-innervation, myotendinous junctions, and revascularization. If a severe injury occurs, the devascularization causes ischemia, while nerve damage may cause atrophic myofibers. If the injury is left untreated, permanent loss of muscle functions may arise [30][31].
Inflammation is a defensive reaction involving microcirculation and it is initiated after injury or musculoskeletal disease. Understanding the process of skeletal muscle inflammation requires an understanding of key aspects of tissue engineering and regenerative medicine. Inflammatory immune responses have evolved to protect the host from invading pathogens and inflammation, along with the damage or death-associated molecular patterns (DAMPs) generated within the body under diverse inflammatory conditions [32]. Macrophages play a central role in inflammatory responses and during muscle wound healing. Generally, an inflammatory immune response is a highly controlled/regulated process that subsides after clearing the inflammation and the process is called resolution of the inflammation [33]. This resolution process involves various anti-inflammatory mechanisms, including the generation of different anti-inflammatory mediators (cytokines, chemokines, and lipid mediators (resolvins, lipoxins, etc.) and differentiation and recruitment of various immune cells with anti-inflammatory functions mainly through alternatively activated macrophages (AAMs) or macrophage (M2) phenotypes. This is supported by a specialized population of regulatory T cells which also infiltrate the injured muscle and promote the M1 to M2 switch to activate the satellite cells for wound healing [34].
If the inflammatory is not resolved, for example, due to the presence of a highly pathogenic organism that was not cleared, or due to endogenous dysregulation of the immune response during sterile inflammatory conditions, it may be dangerous for the host. Following mild muscle, injury tissues go through a series of processes involving inflammation at the site, repair mechanisms, and remodeling [35]. The inflammatory immune response helps in the necrosis and degradation of skeletal muscle. However, if the loss of skeletal muscle or VML overwhelms the repair mechanism over chronic inflammation at the injured site may occur [36]. Towards this, chronic inflammation enhances the gene expression of many inflammatory modulators [37][38]. Among these, the increase in gene expression of the inflammatory cytokines TGF-β and IL-1β have been reported in VML [38]. To overcome this chronic inflammatory response, physical activity might help delay inflammation and improve the regeneration process [39]. Recently, a group of researchers studying the effect of cyclooxygenase (COX) inhibition on biological scaffold mediated repair of VML, found that COX inhibition blocks macrophage differentiation from the M1 to M2-phenotype [40]. The addition of these non-steroidal anti-inflammatory drugs (NSAIDs) for analgesia may interfere with healing in VML patients [9]. In addition to macrophages, various other immune cells help in muscle regeneration. For example, CD8-T along with macrophages help in the secretion of MCP-1 which ultimately aids in the migration of myeloid-derived suppressor cells Gr1 high leading to myoblast proliferation and muscle regeneration [41]. Therefore, an immune response that involves macrophages as a major contributor to muscle regeneration and wound healing requires efforts to minimize the chronic immune response in VML.
3. Role of Biomaterials in VML
Biochemical and physiological phenotypes determine the isolation of MuSCs at different ages and of different embryonic origins. However, it is still a concern that some subpopulations of MuSCs appear at various periods and the relevance of this complexity is not yet known [42][43]. The identification of satellite cells depends upon the expression of the transcription factor paired box 7 (Pax7), as well as anatomical location, but a clear heterogeneity in the population of muscle satellite cells exists. In recent years, various markers, such as CD45, CD11b, Ter119, CD31, and Sca1, have been identified and are used to isolate dormant and activated MuSCs via fluorescence-activated cell sorting (FACS) techniques [44]. Sublaminar satellite cells are sorted due to the transcription factors paired box 3 (Pax3). This factor is only activated in dormant satellite muscle cells, while Myf5 remains inactivated at the injured site. Moreover, all the biomarkers used for the isolation of satellite cells including c-met, CXCR4, and CD34, are familiar to both satellite cells and skeletal muscle tissues [45]. Another important marker which is present in adult satellite cells and during the early development of the muscle laminin receptor is the α7 integrin receptor, which is has a role in the production of ion of the neuromuscular and myotendinous junctions. The ECM and cardiosphere-derived cells (CDCs) are also reported as an ideal candidate for muscle regeneration in VML [46]. Stem cell therapy helps in muscle regeneration, muscular weakness, and muscular dystrophy-associated signs [47][48]. Although extracellular matrix scaffolds are in clinical trials for soft tissue regeneration, cell-based therapy is considered a better approach for VML.
To further improve the efficiency of cell transplantation therapy scientists have combined two different approaches, biological scaffolds and cell therapy, to improve muscle regeneration [49]. In addition, the delivery approach and therapeutic cell source are equally important for the success of cell therapy [50]. Biomaterials used in cell transplantation work as artificial niches must mimic the natural environment [51]. This approach is of great clinical interest and improves engraftment and the survival of implanted cells [52].
Laminin and p38α/β mitogen-activated protein kinase (MAPK) inhibitor-loaded porous hydrogels show positive contributions in the renewal of aged stem cells [53][54]. An interesting cell/hydrogel micromolding methodology was recently applied for designing muscle cells showing 3D structures similar to native tissues [36]. The formulated cell layers were separated from the substrate and could be applied as cell sheets to form multi-layer cell patches or linked to a hydrogel for easy transplantation [55][56]. Hence, hydrogels serve as a suitable candidate for soft tissue regeneration and can help in muscle transplantation in VML.
In muscle regeneration, it is of the utmost importance to understand the mechanism of regeneration in normal tissue. Hence, the main purpose of combining biopolymers, stem cells, and growth factors is to create an artificial niche for muscle regeneration in VML. These synthetic niches follow the natural methods of both differentiation and self-renewal. The local microenvironment of stem cells helps to maintain their identity and regulate their function. The characterization of niches is clear in intestinal crypt stem cells, hematopoietic stem cells, neural stem cells, hair follicle stem cells, and Drosophila germline stem cells [57][58]. The muscle stem cell niche signals are difficult to characterize due to their mechanical, electrical, and chemical properties. The stem cells are located under the basal lamina along with muscle fibers [59][60]. The basal lamina, consisting of collagen, laminin, and proteoglycans, is very important for creating a functional MuSC niche [61]. Moreover, the nourishment of stem cell microvasculature is a vital component of the SC niche and endothelial cells. The finding proves that signals from the host circulation system, muscle fibers, and ECM manage the dormancy, initiation, and proliferation of MuSCs [23][62]. The synthesis of an artificial 3D microenvironment must mimic the natural niche. The microenvironment of MuSCs is polarized in structure and is in the basement membrane and basal lamina. The ideal niche model allows recapitulation of this structure and its amplification in the muscle engineering process to decide the dormant and activation timing of cells [63][64]. In the past, different models have been applied for the in vivo transplantation of artificial niches, such as hydrogels as used to deliver the stem cell to aged, damaged, or diseased tissue sites [51][65]. Moreover, these hydrogel-based biomaterials safely deliver the cells, enhance their viability, and promote the role of endogenous stem cells. These biomaterials also deliver cytokines such as TNF-α, IL-1β, IL-6, IL-8, to enhance the mobilization of endogenous cells, in turn repairing endothelial progenitors and forming blood vessels.
This entry is adapted from the peer-reviewed paper 10.3390/cells10082016
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195.
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13.
- Song, T.; Manoharan, P.; Millay, D.P.; Koch, S.E.; Rubinstein, J.; Heiny, J.A.; Sadayappan, S. Dilated cardiomyopathy-mediated heart failure induces a unique skeletal muscle myopathy with inflammation. Skelet. Muscle 2019, 9, 4.
- Dresser, L.; Wlodarski, R.; Rezania, K.; Soliven, B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J. Clin. Med. 2021, 10, 2235.
- Grogan, B.F.; Hsu, J.R. Volumetric Muscle Loss. J. Am. Acad. Orthop. Surg. 2011, 19, S35–S37.
- Schnall, B.L.; Chen, M.Y.-T.; Bell, E.M.; Wolf, E.J.; Wilken, J.M. Functional Outcomes of Service Members With Bilateral Transfemoral and Knee Disarticulation Amputations Resulting From Trauma. Mil. Med. 2016, 181, 55–60.
- Greising, S.M.; Dearth, C.L.; Corona, B.T. Regenerative and Rehabilitative Medicine: A Necessary Synergy for Functional Recovery from Volumetric Muscle Loss Injury. Cells Tissues Organs 2016, 202, 237–249.
- Ziemkiewicz, N.; Hilliard, G.; Pullen, N.; Garg, K. The Role of Innate and Adaptive Immune Cells in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2021, 22, 3265.
- Goldman, S.M.; Valerio, M.S.; Janakiram, N.B.; Dearth, C.L. COX-2 inhibition does not alter wound healing outcomes of a volumetric muscle loss injury treated with a biologic scaffold. J. Tissue Eng. Regen. Med. 2020, 14, 1929–1938.
- Magarotto, F.; Sgro, A.; Dorigo Hochuli, A.H.; Andreetta, M.; Grassi, M.; Saggioro, M.; Nogara, L.; Tolomeo, A.M.; Francescato, R.; Collino, F.; et al. Muscle functional recovery is driven by extracellular vesicles combined with muscle extracellular matrix in a volumetric muscle loss murine model. Biomaterials 2021, 269, 120653.
- Goldman, S.M.; Janakiram, N.B.; Valerio, M.S.; Dearth, C.L. Evaluation of licofelone as an adjunct anti-inflammatory therapy to biologic scaffolds in the treatment of volumetric muscle loss. Cell Tissue Res. 2021, 1–11.
- Rodriguez, B.L.; Vega-Soto, E.E.; Kennedy, C.S.; Nguyen, M.H.; Cederna, P.S.; Larkin, L.M. A tissue engineering approach for repairing craniofacial volumetric muscle loss in a sheep following a 2, 4, and 6-month recovery. PLOS ONE 2020, 15, e0239152.
- Nuge, T.; Tshai, K.Y.; Lim, S.S.; Nordin, N.; Hoque, E. Characterization and optimization of the mechanical properties of electrospun gelatin nanofibrous scaffolds. World J. Eng. 2020, 17, 12–20.
- Shayan, M.; Huang, N.F. Pre-Clinical Cell Therapeutic Approaches for Repair of Volumetric Muscle Loss. Bioeng. 2020, 7, 97.
- Sciorati, C.; Rigamonti, E.; Manfredi, A.A.; Rovere-Querini, P. Cell death, clearance and immunity in the skeletal muscle. Cell Death Differ. 2016, 23, 927–937.
- Collins, R.A.; Grounds, M.D. The Role of Tumor Necrosis Factor-alpha (TNF-α) in Skeletal Muscle Regeneration: Studies in TNF-α(-/-) and TNF-α(-/-)/LT-α(-/-) Mice. J. Histochem. Cytochemi. 2001, 49, 989–1001.
- Frenette, J.; Cai, B.; Tidball, J.G. Complement Activation Promotes Muscle Inflammation during Modified Muscle Use. Am. J. Pathol. 2000, 156, 2103–2110.
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, R1173–R1187.
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801.
- Bazgir, B.; Fathi, R.; Valojerdi, M.R.; Mozdziak, P.; Asgari, A. Satellite Cells Contribution to Exercise Mediated Muscle Hypertrophy and Repair. Cell J 2016, 18, 473–484.
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage Polarization in Inflammatory Diseases. Int. J. Biol. Sci. 2014, 10, 520–529.
- Etienne, J.; Liu, C.; Skinner, C.M.; Conboy, M.J.; Conboy, I.M. Skeletal muscle as an experimental model of choice to study tissue aging and rejuvenation. Skelet. Muscle 2020, 10, 1–16.
- Yin, H.; Price, F.; Rudnicki, M. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67.
- Yablonka-Reuveni, Z. The skeletal muscle satellite cell: Still young and fascinating at 50. J. Histochem. Cytochem. 2011, 59, 1041–1059.
- Saclier, M.; Theret, M.; Mounier, R.; Chazaud, B. Effects of Macrophage Conditioned-Medium on Murine and Human Muscle Cells: Analysis of Proliferation, Differentiation, and Fusion. Methods Mol. Biol. 2017, 1556, 317–327.
- Bencze, M.; Negroni, E.; Vallese, D.; Yacoub-Youssef, H.; Chaouch, S.; Wolff, A.; Aamiri, A.; Di Santo, J.; Chazaud, B.; Butler-Browne, G.; et al. Proinflammatory Macrophages Enhance the Regenerative Capacity of Human Myoblasts by Modifying Their Kinetics of Proliferation and Differentiation. Mol. Ther. 2012, 20, 2168–2179.
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; Van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069.
- Dort, J.; Fabre, P.; Molina, T.; Dumont, N.A. Macrophages Are Key Regulators of Stem Cells during Skeletal Muscle Regeneration and Diseases. Stem Cells Int. 2019, 2019, 1–20.
- Christ, G.J.; Saul, J.M.; Furth, M.E.; Andersson, K.-E. The Pharmacology of Regenerative Medicine. Pharmacol. Rev. 2013, 65, 1091–1133.
- Cezar, C.A.; Mooney, D.J. Biomaterial-based delivery for skeletal muscle repair. Adv. Drug Deliv. Rev. 2015, 84, 188–197.
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330.
- Kumar, V. Inflammation research sails through the sea of immunology to reach immunometabolism. Int. Immunopharmacol. 2019, 73, 128–145.
- Fullerton, J.; Gilroy, D. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567.
- Schiaffino, S.; Pereira, M.G.; Ciciliot, S.; Rovere-Querini, P. Regulatory T cells and skeletal muscle regeneration. FEBS J. 2016, 284, 517–524.
- Caseiro, A.; Pereira, T.; Bartolo, P.; Santos, J.; Luís, A.L.; Maurício, A.C.; Santos, A.R.C. Mesenchymal Stem Cells and Biomaterials Systems—Perspectives for Skeletal Muscle Tissue Repair and Regeneration. Procedia Eng. 2015, 110, 90–97.
- Kim, H.; Bae, C.; Kook, Y.-M.; Koh, W.-G.; Lee, K.; Park, M.H. Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Res. Ther. 2019, 10, 1–14.
- Nuutila, K.; Sakthivel, D.; Kruse, C.; Tran, P.; Giatsidis, G.; Sinha, I. Gene expression profiling of skeletal muscle after volumetric muscle loss. Wound Repair Regen. 2017, 25, 408–413.
- Corona, B.T.; Rivera, J.C.; Greising, S.M. Inflammatory and Physiological Consequences of Debridement of Fibrous Tissue after Volumetric Muscle Loss Injury. Clin. Transl. Sci. 2017, 11, 208–217.
- Kim, J.T.; Kasukonis, B.; Dunlap, G.; Perry, R.; Washington, T.; Wolchok, J.C.; Wolchok, J. Regenerative Repair of Volumetric Muscle Loss Injury is Sensitive to Age. Tissue Eng. Part A 2020, 26, 3–14.
- Nakanishi, Y.; Nakatsuji, M.; Seno, H.; Ishizu, S.; Akitake-Kawano, R.; Kanda, K.; Ueo, T.; Komekado, H.; Kawada, M.; Minami, M.; et al. COX-2 inhibition alters the phenotype of tumor-associated macrophages from M2 to M1 in ApcMin/+ mouse polyps. Carcinogenesis 2011, 32, 1333–1339.
- Zhang, J.; Xiao, Z.; Qu, C.; Cui, W.; Wang, X.; Du, J. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and Gr1(high) macrophage infiltration. J. Immunol. 2014, 193, 5149–5160.
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells—Current trends and future prospective. Biosci. Rep. 2015, 35, e00191.
- Wilson, A.; Hodgson-Garms, M.; Frith, J.E.; Genever, P. Multiplicity of Mesenchymal Stromal Cells: Finding the Right Route to Therapy. Front. Immunol. 2019, 10, 1112.
- Maesner, C.C.; Almada, A.E.; Wagers, A.J. Established cell surface markers efficiently isolate highly overlapping populations of skeletal muscle satellite cells by fluorescence-activated cell sorting. Skelet. Muscle 2016, 6, 1–10.
- Francetic, T.; Li, Q. Skeletal myogenesis andMyf5activation. Transcription 2011, 2, 109–114.
- Rogers, R.G.; Li, L.; Peck, K.; Sanchez, L.; Liu, W.; Ciullo, A.; Alfaro, J.; Rannou, A.; Fournier, M.; Lee, Y.; et al. Cardiosphere-derived cells, with and without a biological scaffold, stimulate myogenesis and recovery of muscle function in mice with volumetric muscle loss. Biomaterials 2021, 274, 120852.
- Nalbandian, M.; Zhao, M.; Sasaki-Honda, M.; Jonouchi, T.; Lucena-Cacace, A.; Mizusawa, T.; Yasuda, M.; Yoshida, Y.; Hotta, A.; Sakurai, H. Characterization of hiPSC-Derived Muscle Progenitors Reveals Distinctive Markers for Myogenic Cell Purification Toward Cell Therapy. Stem Cell Rep. 2021, 16, 883–898.
- Zhao, M.; Tazumi, A.; Takayama, S.; Takenaka-Ninagawa, N.; Nalbandian, M.; Nagai, M.; Nakamura, Y.; Nakasa, M.; Watanabe, A.; Ikeya, M.; et al. Induced Fetal Human Muscle Stem Cells with High Therapeutic Potential in a Mouse Muscular Dystrophy Model. Stem Cell Rep. 2020, 15.
- Rizzi, R.; Bearzi, C.; Mauretti, A.; Bernardini, S.; Cannata, S.; Gargioli, C. Tissue engineering for skeletal muscle regeneration. Muscle Ligaments Tendons J. 2012, 2, 230–234.
- Urciuolo, A.; De Coppi, P. Decellularized Tissue for Muscle Regeneration. Int. J. Mol. Sci. 2018, 19, 2392.
- Lutolf, M.P.; Gilbert, P.; Blau, H.M. Designing materials to direct stem-cell fate. Nat. Cell Biol. 2009, 462, 433–441.
- Lutolf, M.P.; Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55.
- Jones, N.C.; Tyner, K.J.; Nibarger, L.; Stanley, H.M.; Cornelison, D.D.W.; Fedorov, Y.V.; Olwin, B.B. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J. Cell Biol. 2005, 169, 105–116.
- Cosgrove, B.D.; Gilbert, P.M.; Porpiglia, E.; Mourkioti, F.; Lee, S.P.; Corbel, S.Y.; Llewellyn, M.E.; Delp, S.L.; Blau, H.M. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 2014, 20, 255–264.
- Hasan, A.; Paul, A.; Vrana, N.E.; Zhao, X.; Memic, A.; Hwang, Y.-S.; Dokmeci, M.R.; Khademhosseini, A. Microfluidic techniques for development of 3D vascularized tissue. Biomaterials 2014, 35, 7308–7325.
- Zorlutuna, P.; Annabi, N.; Camci-Unal, G.; Nikkhah, M.; Cha, J.M.; Nichol, J.W.; Manbachi, A.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabricated Biomaterials for Engineering 3D Tissues. Adv. Mater. 2012, 24, 1782–1804.
- Dash, B.C.; Xu, Z.; Lin, L.; Koo, A.; Ndon, S.; Berthiaume, F.; Dardik, A.; Hsia, H. Stem Cells and Engineered Scaffolds for Regenerative Wound Healing. Bioengineering 2018, 5, 23.
- Wu, R.-X.; Xu, X.-Y.; Wang, J.; He, X.-T.; Sun, H.-H.; Chen, F.-M. Biomaterials for endogenous regenerative medicine: Coaxing stem cell homing and beyond. Appl. Mater. Today 2018, 11, 144–165.
- Ferraro, F.; Celso, C.L.; Scadden, D. Adult stem cels and their niches. Adv. Exp. Med. Biol. 2010, 695, 155–168.
- Morrison, S.J.; Spradling, A.C. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell 2008, 132, 598–611.
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2506–2519.
- Rossi, C.A.; Pozzobon, M.; De Coppi, P. Advances in musculoskeletal tissue engineering: Moving towards therapy. Organogenesis 2010, 6, 167–172.
- Cruz-Acuña, R.; García, A.J. Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. J. Int. Soc. Matrix Biol. 2017, 57–58, 324–333.
- Thomas, K.; Engler, A.; Meyer, G.A. Extracellular matrix regulation in the muscle satellite cell niche. Connect. Tissue Res. 2014, 56, 1–8.
- Donnelly, H.; Salmeron-Sanchez, M.; Dalby, M.J. Designing stem cell niches for differentiation and self-renewal. J. R. Soc. Interface 2018, 15, 20180388.
