The unique biology of the intestinal epithelial barrier is linked to a low baseline oxygen pressure (pO2), characterised by a high rate of metabolites circulating through the intestinal blood and the presence of a steep oxygen gradient across the epithelial surface. These characteristics require tight regulation of oxygen homeostasis, achieved in part by hypoxia-inducible factor (HIF)-dependent signalling. Furthermore, intestinal epithelial cells (IEC) possess metabolic identities that are reflected in changes in mitochondrial function. In recent years, it has become widely accepted that oxygen metabolism is key to homeostasis at the mucosae. In addition, the gut has a vast and diverse microbial population, the microbiota. Microbiome–gut communication represents a dynamic exchange of mediators produced by bacterial and intestinal metabolism. The microbiome contributes to the maintenance of the hypoxic environment, which is critical for nutrient absorption, intestinal barrier function, and innate and/or adaptive immune responses in the gastrointestinal tract.
- oxygen
- hypoxia
- microbiota
1. Introduction
2. Oxygen Scenery at the Intestinal Epithelial Barrier
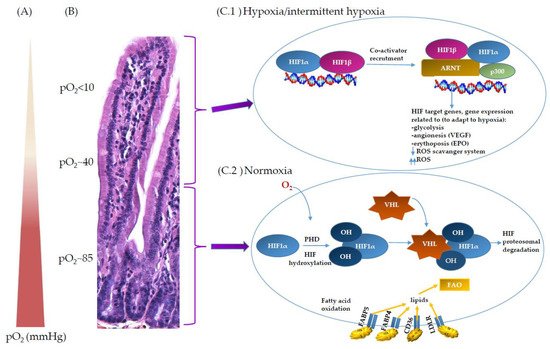
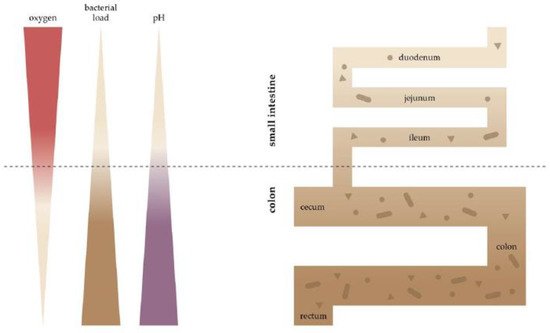
3. Oxygen Sensing at the Epithelial Barrier in IBD
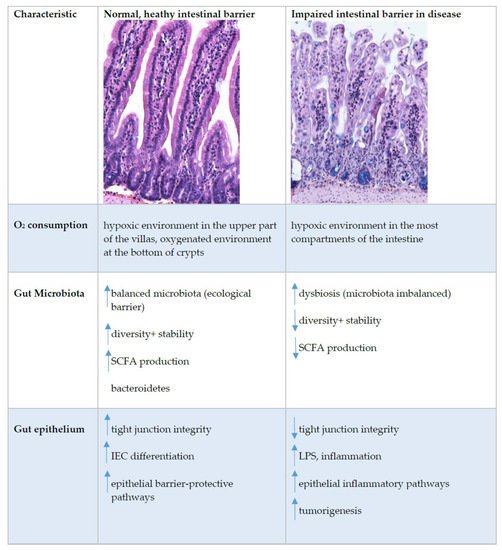
This entry is adapted from the peer-reviewed paper 10.3390/ijms22179170
References
- Taylor, C.T.; Colgan, S.P. Hypoxia and gastrointestinal disease. J. Mol. Med. 2007, 85, 1295–1300.
- Rath, E.; Haller, D. Inflammation and cellular stress: A mechanistic link between immune-mediated and metabolically driven pathologies. Eur. J. Nutr. 2011, 50, 219–233.
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33.
- Rath, E.; Moschetta, A.; Haller, D. Mitochondrial function—Gatekeeper of intestinal epithelial cell homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 497–516.
- Colgan, S.P.; Taylor, C.T. Hypoxia: An alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 281–287.
- Karhausen, J.; Haase, V.H.; Colgan, S.P. Inflammatory hypoxia: Role of hypoxia-inducible factor. Cell Cycle 2005, 4, 256–258.
- McElroy, G.S.; Chandel, N.S. Mitochondria control acute and chronic responses to hypoxia. Exp. Cell Res. 2017, 356, 217–222.
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balazsi, S.; Hajnady, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 2018, 9, 105.
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671.
- Albenberg, L.; Esipova, T.V.; Judge, C.P.; Bittinger, K.; Chen, J.; Laughlin, A.; Grunberg, S.; Baldassano, R.N.; Lewis, J.D.; Li, H.; et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 2014, 147, 1055–1063.
- Calcagno, M.; Kahleova, H.; Alwarith, J.; Burgess, N.N.; Flores, R.A.; Busta, M.L.; Barnard, N.D. The Thermic Effect of Food: A Review. J. Am. Coll. Nutr. 2019, 38, 547–551.
- Zheng, L.; Kelly, C.J.; Colgan, S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, C350–C360.
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32.
- Singhal, R.; Shah, Y.M. Oxygen battle in the gut: Hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 2020, 295, 10493–10505.
- Zweier, J.L.; He, G.; Samouilov, A.; Kuppusamy, P. EPR spectroscopy and imaging of oxygen: Applications to the gastrointestinal tract. Adv. Exp. Med. Biol. 2003, 530, 123–131.
- He, G.; Shankar, R.A.; Chzhan, M.; Samouilov, A.; Kuppusamy, P.; Zweier, J.L. Noninvasive measurement of anatomic structure and intraluminal oxygenation in the gastrointestinal tract of living mice with spatial and spectral EPR imaging. Proc. Natl. Acad. Sci. USA 1999, 96, 4586–4591.
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial pressure of oxygen in the human body: A general review. Am. J. Blood Res. 2019, 9, 1–14.
- Shah, Y.M. The role of hypoxia in intestinal inflammation. Mol. Cell. Pediatrics 2016, 3, 1.
- De Souza, H.S.P.; Fiocchi, C.; Iliopoulos, D. The IBD interactome: An integrated view of aetiology, pathogenesis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 739–749.
- Li, X.; Egervari, G.; Wang, Y.; Berger, S.L.; Lu, Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Biol. 2018, 19, 563–578.
- Glover, L.E.; Colgan, S.P. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology 2011, 140, 1748–1755.
- Ledoux, S.; Runembert, I.; Koumanov, K.; Michel, J.B.; Trugnan, G.; Friedlander, G. Hypoxia enhances Ecto-5′-Nucleotidase activity and cell surface expression in endothelial cells: Role of membrane lipids. Circ. Res. 2003, 92, 848–855.
- Van Welden, S.; Selfridge, A.C.; Hindryckx, P. Intestinal hypoxia and hypoxia-induced signalling as therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 596–611.
- Campbell, E.L.; Bruyninckx, W.J.; Kelly, C.J.; Glover, L.E.; McNamee, E.N.; Bowers, B.E.; Bayless, A.J.; Scully, M.; Saeedi, B.J.; Golden-Mason, L.; et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 2014, 40, 66–77.
- Kelly, C.J.; Glover, L.E.; Campbell, E.L.; Kominsky, D.J.; Ehrentraut, S.F.; Bowers, B.E.; Bayless, A.J.; Saeedi, B.J.; Colgan, S.P. Fundamental role for HIF-1alpha in constitutive expression of human beta defensin-1. Mucosal Immunol. 2013, 6, 1110–1118.
- Perler, B.K.; Ungaro, R.; Baird, G.; Mallette, M.; Bright, R.; Shah, S.; Shapiro, J.; Sands, B.E. Presenting symptoms in inflammatory bowel disease: Descriptive analysis of a community-based inception cohort. BMC Gastroenterol. 2019, 19, 47.
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The impact of the level of the intestinal short chain Fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem. J. 2010, 4, 53–58.
- Rivera-Chavez, F.; Lopez, C.A.; Baumler, A.J. Oxygen as a driver of gut dysbiosis. Free Radic. Biol. Med. 2017, 105, 93–101.
- Chang, C.S.; Kao, C.Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019, 26, 59.
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608.
- Chun, C.; Zheng, L.; Colgan, S.P. Tissue metabolism and host-microbial interactions in the intestinal mucosa. Free Radic. Biol. Med. 2017, 105, 86–92.
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785.
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119.
- Ritzhaupt, A.; Wood, I.S.; Ellis, A.; Hosie, K.B.; Shirazi-Beechey, S.P. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: Its potential to transport L-lactate as well as butyrate. J. Physiol. 1998, 513, 719–732.
- Thibault, R.; De Coppet, P.; Daly, K.; Bourreille, A.; Cuff, M.; Bonnet, C.; Mosnier, J.F.; Galmiche, J.P.; Shirazi-Beechey, S.; Segain, J.P. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology 2007, 133, 1916–1927.
- Cresci, G.; Nagy, L.E.; Ganapathy, V. Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. JPEN. J. Parenter. Enter. Nutr. 2013, 37, 763–774.
- Fachi, J.L.; Felipe, J.S.; Pral, L.P.; da Silva, B.K.; Correa, R.O.; de Andrade, M.C.P.; da Fonseca, D.M.; Basso, P.J.; Camara, N.O.S.; de Sales, E.S.E.L.; et al. Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. 2019, 27, 750–761.
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10.
- Jackson, D.N.; Theiss, A.L. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes 2020, 11, 285–304.
- Ott, S.J.; Musfeldt, M.; Wenderoth, D.F.; Hampe, J.; Brant, O.; Folsch, U.R.; Timmis, K.N.; Schreiber, S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004, 53, 685–693.
