The ongoing global coronavirus-19 disease (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), poses major challenges to health systems worldwide. While the majority of infected people have mild to moderate symptoms, some patients develop acute respiratory distress syndrome (ARDS) requiring intensive care treatment and mechanical ventilation.
- SARS-CoV-2 virus variant
- Alpha variant
- COVID-19
- intensive care medicine
- mortality
1. Introduction
SARS-CoV-2 is a positive-sense single-stranded RNA virus whose genome is of a low stability and is thus more prone to mutation accumulation, with approximately 9.8 × 104 substitutions/site yearly [1][2][3][4]. By the beginning of May 2021, there had been more than 1.4 million sequences reported, and among them, 3913 major representative variant genomes that have been identified and included in the global SARS-CoV-2 sequence database operated by Global Initiative on Sharing Avian Influenza Data (GISAID). Not all genetic mutations lead to variation in major proteins and/or alter virus infectivity. In the meantime, several mutations of SARS-CoV-2 have emerged . These genetic variants affect the course of the disease by altered virulence, susceptibility to immune response and transmissibility. Four of these variants are classified as variants of concern (VOC) by the World Health Organization (WHO): Alpha variant/Lineage B.1.1.7 (first detected in the UK) [5], Beta variant/Lineage B.1.351 (first detected in South Africa) , Gamma variant/Lineage P.1 (first detected in Brazil)[6][7] and Delta variant/Lineage B.1.617.2 (first detected in India) [8]. The current spike gene mutations account for most of the clinically influential VOC.
To date, the pandemic has evolved in waves with rapidly increasing infections and deaths in most countries. These waves led to various measures, such as lockdowns, mandatory masks and others, which consequently resulted in decreasing infection rates.
In Germany, the second COVID-19 wave in the autumn of 2020 was still caused by the SARS-CoV-2 wild-type (WT), but the Alpha variant successively superseded the WT and constituted the predominant COVID-19 pathogen from March 2021 onwards. The SARS-CoV-2 Alpha variant (lineage B.1.1.7) was first detected in the UK in September 2020 [5] and was shortly after named the Alpha variant. The Alpha variant has an N501Y mutation: at the 501 residue, asparagine N has been replaced with Y tyrosine, and K417N, lysine K, has been replaced with asparagine N. Evaluations[9] of the Robert Koch Institute illustrated a continuous increase in the proportion of infections with the Alpha variant up to more than 90% at the end of April 2021 [10]. Previous studies described the Alpha variant as being significantly more contagious. Evidence suggests that the VOC Alpha increased the transmissibility rate by ~50%, especially in younger age groups and children[11]; however, data regarding the severity of disease when compared to the SARS-CoV-2 WT are inconclusive [12][13][14][15][16][17]. At present, very limited data are available regarding the course of patients requiring admission to intensive care units (ICU) and the impact of the Alpha variant on ICU mortality[17].
Therefore, the aim of this study was to analyze the outcome and clinical course of patients with Alpha variant SARS-CoV-2 infections in the ICU of a maximum care hospital in Germany and to compare it to patients with WT infections.
2. Results
2.1. Baseline Characteristics of Full Patient Population (n = 160)
| Full Patient Population (n = 160) | Matched-Pair Analysis (n = 116) | |||||
|---|---|---|---|---|---|---|
| Alpha Variant (n = 80) | Wild Type (n = 80) | p | Alpha Variant (n = 58) | Wild Type (n = 58) | p | |
| Age (median, range) | 55.5 (13–83) | 62.5 (16–87) | 0.073 | 61 (26–83) # | 62 (25–85) # | 0.917 |
| Males | 59/80 (74%) | 57/80 (71%) | 0.860 | 43/58 (74.1%) # | 43/58 (74.1%) # | 1 |
| Diabetes | 25/80 (26%) | 21/80 (31%) | 0.601 | 20/58 (34.5%) | 14/58 (24.1%) | 0.308 |
| Chronic respiratory diseases | 13/80 (16%) | 10/80 (13%) | 0.653 | 9/58 (15.5%) | 4/58 (6.9%) | 0.238 |
| Cardiovascular disease | 44/80 (55%) | 40/80 (50%) | 0.635 | 30/58 (51.7%) | 30/58 (51.7%) | 1 |
| Oncologic/hematologic disease/immunosuppressive medication | 11/80 (14%) | 16/80 (20%) | 0.399 | 9/58 (15.5%) | 12/58 (20.7%) | 0.630 |
| Liver disease | 8/80 (10%) | 4/80 (5%) | 0.369 | 5/58 (8.6%) | 3/58 (5.2%) | 0.717 |
3.2. ICU Procedures and Outcomes in Full Patient Population (n = 160)
| Full Patient Population (n = 160) | Matched-Pair Analysis (n = 116) | |||||
|---|---|---|---|---|---|---|
| Alpha (n = 80) | Wild Type (n = 80) | p | Alpha (n = 58) | Wild Type (n = 58) | p | |
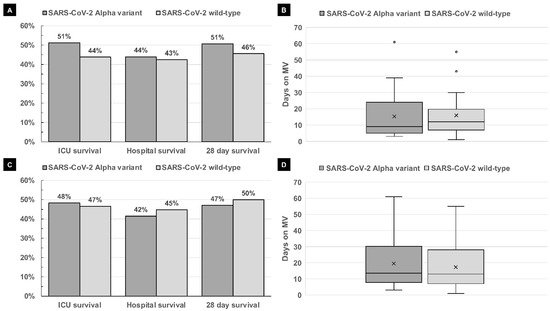
| ICU survival | 41/80 (51%) | 35/80 (44%) | 0.429 | 28/58 (48%) | 27/58 (47%) | 1 |
| Hospital survival | 33/75 (44%) | 34/80 (43%) | 0.872 | 22/53 (42%) | 26/58 (45%) | 0.848 |
| 28-day survival | 36/71 (51%) | 36/79 (46%) | 0.624 | 24/51 (47.1%) | 29/58 (50%) | 0.848 |
| Mechanical ventilation | 61/80 (76%) | 52/80 (65%) | 0.165 | 45/58 (78%) | 38/58 (66%) | 0.217 |
| Duration of mechanical ventilation (days) (median, range) |
16 (2–61) | 14 (1–55) | 0.814 | 16 (2–61) | 14.5 (1–55) | 0.694 |
| Duration of mechanical ventilation of ICU survivors (days) (median, range) | 11 (3–61) [n = 23] | 12 (1–55) [n = 18] | 0.814 | 13.5 (3–61) [n = 28] | 13 (1–55) [n = 15] | 0.694 |
| Duration of NIV/HFNO in patients with no MV (days) (median, range) |
6 (2–14) [n= 16] | 5.5 (1–20) [n = 16] | 0.934 | 5 (2–9) [n = 11] | 4 (1–13) [n = 11] | 0.844 |
| ECMO | 6/80 (8%) | 14/79 (18%) | 0.059 | 10/58 (17%) | 6/58 (10%) | 0.420 |
| Renal replacement therapy | 17/77 (22%) | 23/80 (29%) | 0.365 | 13/56 (23%) | 17/58 (29%) | 0.526 |
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms9091944
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020. Available online: http://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 (accessed on 6 September 2021).
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- van Dorp, L.; Acman, M.; Richard, D.; Shaw, L.P.; Ford, C.E.; Ormond, L.; Owen, C.J.; Pang, J.; Tan, C.C.S.; Boshier, F.A.T.; et al. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect. Genet. Evol. 2020, 83, 104351. [Google Scholar] [CrossRef] [PubMed]
- Public Health England Investigation of Novel SARS-CoV-2 Variant Variant of Concern 202012/01 Detection of an Epidemiological Cluster Associated with a New Variant of Concern Nomenclature of Variants in the UK Current Epidemiological Findings. 2020. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/959361/Technical_Briefing_VOC202012-2_Briefing_2.pdf (accessed on 6 September 2021).
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Emergence of a SARS-CoV-2 variant of concern with mutations in spike glycoprotein. Nature 2021, 592, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Ramundo, M.S.; De Jesus, J.G.; Andrade, P.S.; Coletti, T.M. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil|Enhanced Reader. Science 2021, 821, eabh2644. [Google Scholar]
- European Centre for Disease Prevention and Control Emergence of SARS-CoV-2 B.1.617 Variants in India and Situation in the EU/EEA Event Background Epidemiology. 2021. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Emergence-of-SARS-CoV-2-B.1.617-variants-in-India-and-situation-in-the-EUEEA_0.pdf (accessed on 6 September 2021).
- GISAID hCov19 Variants. Available online: https://www.gisaid.org/hcov19-variants/ (accessed on 6 June 2021).
- Robert Koch Institut Bericht zu Virusvarianten Von SARS-CoV-2 in Deutschland. 2021. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/DESH/Bericht_VOC_2021-07-14.pdf?__blob=publicationFile (accessed on 6 September 2021).
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, Á.; et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature 2021, 593, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Jarvis, C.I.; van Zandvoort, K.; Clifford, S.; Sun, F.Y.; Funk, S.; Medley, G.; Jafari, Y.; Meakin, S.R.; Lowe, R.; et al. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature 2021, 593, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Grint, D.J.; Wing, K.; Williamson, E.; McDonald, H.I.; Bhaskaran, K.; Evans, D.; Evans, S.J.W.; Walker, A.J.; Hickman, G.; Nightingale, E.; et al. Case fatality risk of the SARS-CoV-2 variant of concern B.1.1.7 in England, 16 November to 5 February. Eurosurveillance 2021, 26, 2100256. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Merchant, H.A.; Hasan, S.S. Mortality risk in patients infected with SARS-CoV-2 of the lineage B.1.1.7 in the UK. J. Infect. 2021, 83, e14–e15. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef] [PubMed]
- Challen, R.; Brooks-Pollock, E.; Read, J.M.; Dyson, L.; Tsaneva-Atanasova, K.; Danon, L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: Matched cohort study. BMJ 2021, n579. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, L.; Espinel, M.A.; Abreu, M.; González-Alba, J.M.; Gijón, D.; McGee, A.; Cantón, R.; Galán, J.C.; Aranaz, J. Emergence and Spread of B.1.1.7 Lineage in Primary Care and Clinical Impact in the Morbi-Mortality among Hospitalized Patients in Madrid, Spain. Microorganisms 2021, 9, 1517. [Google Scholar] [CrossRef] [PubMed]
