Protein-based subunit nanovaccines are typically composed of native or altered protein antigens that can self-assemble into nanoparticles, or antigens associated with nanoparticles through covalent or noncovalent interactions. Characteristically, nanovaccines are 1 to 1000 nm in size which generally facilitates the induction of stronger immune responses.
- Nanoparticles
- Conjugates
- Nanovaccines
- Subunit vaccines
- Protein vaccines
1. Introduction
Traditional whole-pathogen vaccines utilize whole microorganisms, such as viruses and bacteria, which may be live-attenuated or killed. The utility of vaccines that contain living pathogenic microorganisms is constrained by the requirement for refrigerated transport to preserve potency, and the chance that the pathogens could revert to their virulent forms under particular conditions[1]. Inactivated vaccines, based on killed pathogens, do not usually require refrigeration and are transported in dried forms; however, these vaccines induce weaker immune responses and booster doses may be needed to maintain immune response potency[2]. Unfortunately, traditional whole-pathogen vaccines may induce extensive inflammation, allergies, and autoimmune responses. The drawbacks of traditional vaccines have limited their applications[3]. Albeit, traditional vaccines are still widely used in veterinary science as the safety criteria for veterinary vaccines are far less strict compared to those implemented for human use[4].
Subunit vaccines contain purified or recombinant components derived from a pathogen, such as polysaccharides, peptides, or proteins that have antigenic properties[5][6]. In comparison to their whole-pathogen counterparts, subunit vaccines cause minimal adverse effects, do not require complex storage or transport conditions, and have large-scale manufacturing potential[7][8][9]. However, subunit vaccines, or more precisely subunit antigens, are often unable to trigger a strong immune response and, therefore, require the application of molecular adjuvants or delivery systems to boost immunity[10][11][12][13]. Of the various adjuvants and delivery systems in development over the past few decades, nanoparticles have been extensively investigated for enhancing the efficacy of subunit vaccines.
Nanoparticles (NPs) resemble the size of natural pathogens, such as viruses and nanobacteria, making them easily recognizable by immune cells; especially antigen-presenting cells (APCs). APCs are part of innate immunity and serve as the crucial linker/activator that triggers adaptive immune responses. Although some amphipathic proteins (e.g., ferritins) or peptides may self-assemble into NPs by themselves, most need to be associated with nanomaterials through covalent or noncovalent interactions to form NPs. Peptide antigens can be associated with protein carriers that have self-assembly properties to form protein-based subunit nanovaccines; while protein antigens can be associated with NPs, including lipid NPs, polymeric NPs, protein NPs, or inorganic NPs, to form protein-based subunit nanovaccine.
Covalent linkage between an antigen and a nanoparticulate delivery system is normally stronger than noncovalent linkage. Consequently, covalently linked systems are more stable under complex in vivo conditions, and conjugation techniques are often applied to produce nanovaccines (Figure 1)[14]. In some cases, where proteins function as nanocarriers, recombinant protein expression can be applied to express fused proteins to achieve covalent linkage.
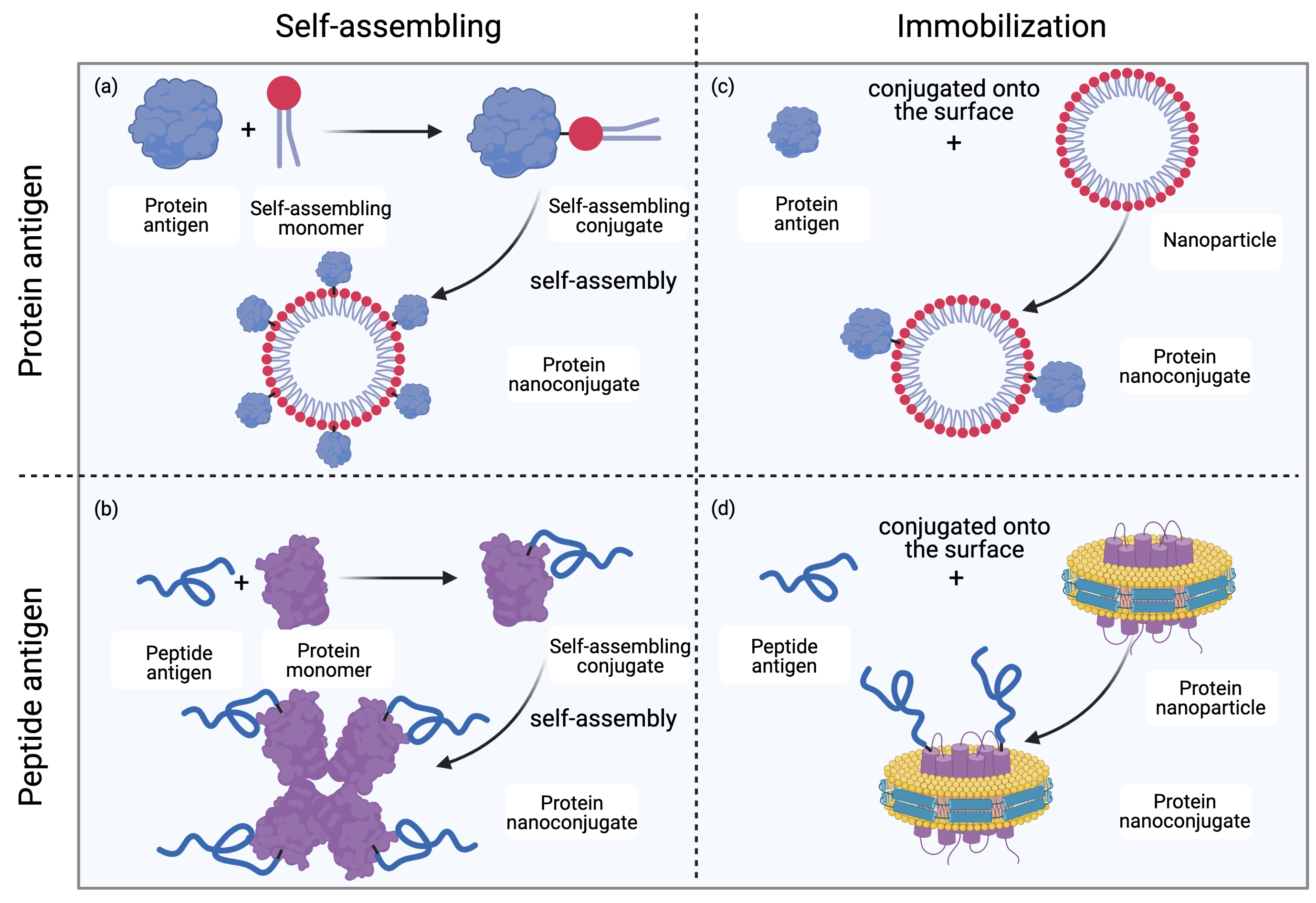
Figure 1. Methods for protein nanoconjugate formation. (a) Protein antigens are conjugated to self-assembling monomers; the conjugates then self-assemble into protein nanoconjugates. (b) Peptide antigens are conjugated to protein monomers; the conjugates then self-assemble into protein nanoconjugates. (c) Protein antigens are conjugated to the surface of nanoparticles (NPs) to form protein nanoconjugates. (d) Peptide antigens are conjugated to the surface of NPs (for example, lipoprotein NPs) to form protein nanoconjugates. Drawing created with Biorender.com.
2. Preparation of Protein Nanovaccines
A wide variety of compositions have been investigated to produce protein-based subunit nanovaccines. Depending on the carriers and immunogens, such vaccines can be divided into protein carrier-based, lipid-based, polymer-based and inorganic particle-based nanovaccines[14][15].
2.1 Protein carrier-based nanovaccines
Virus-like particles (VLPs) are one of the most successful subclasses of protein carrier-based nanovaccines developed to date. VLPs are made up of viral proteins that have the ability to self-assemble into nanoscale particles. Several vaccines based on VLPs targeting human papilloma virus, hepatitis B virus, and malaria have been approved by the FDA, including Gardasil®, Gardasil9®, Cervarix®, Sci-B-VacTM, and MosquirixTM[16]. These proteins not only function as immunogens and carriers, but can also be fused with a variety of non-viral epitopes to induce desired immune responses.
Non-viral proteins have also been reported to have the ability to self-assemble. Cage-like proteins, such as E2 protein derived from the pyruvate dehydrogenase complex of Bacillus stearothermophilus, can self-assemble into 25 nm particles consisting of 60 identical monomers[17]. Chemical methods have been applied to conjugate different antigens onto the surface of E2 protein to obtain protein subunit nanovaccines[17][18][19]. The produced nanoparticles co-delivered antigens and CpG molecules eventually elicited stronger antitumor immune responses compared to the antigens administered with CpG, providing the rationale of using E2 as a protein carrier for vaccine development. Vault proteins isolated from the cytoplasm of eukaryotic cells can also self-assemble into a cage-like barrel-shaped structure (30/60 nm)[20]. Several antigens have been fused with vaults to boost antigen-specific immune responses without the need for an additional adjuvant[21][22][23][24]. Apart from spherical protein carriers, protein carriers with a variety of other morphologies, such as nanofiber and nanodisc, have also been investigated to formulate protein nanovaccines[25][26].
2.2 Lipid-based nanovaccines
Lipidic delivery systems, especially liposomes and lipid NPs, have been extensively used in clinics to combat cancer and infectious diseases[27][28][29]. Liposomes have an aqueous core that is trapped by single or multiple bilayers consisting of natural or synthetic lipids[30]. Although antigens are typically delivered by liposomes in encapsulated form[31], it has been repeatedly demonstrated that covalent linkage of antigens and liposomes, referred to as antigen anchorage onto liposomes, can induce more robust immune responses compared to antigens alone or antigens associated with liposomes through noncovalent interactions[32][33][34]. For example, lipidated and non-lipidated Group A Streptococcus M-protein-derived antigens were formulated into liposomes for intranasal vaccine delivery[34]. Liposomes with covalently anchored antigen (lipidated) elicited a stronger humoral response by producing higher titers of antigen-specific IgG in comparison to the antigen (non-lipidated) encapsulated in liposomes.
2.3 Polymer-based protein nanovaccines
Like lipidic NPs, polymeric NPs are also popular as nanovaccine delivery systems. In many cases, antigens have been encapsulated within the core of polymer NPs, or associated with polymer NPs through electrostatic interactions by opposite charges[35][36][37]. However, as discussed previously, such noncovalent interactions are generally weaker than covalent linkages. Chemical methods have been applied to conjugate a variety of protein antigens with polymeric material, such as poly(glutamic acid), N-trimethylaminoethylmethacrylate chitosan, carboxylated polystyrene, and pluronic-stabilized poly(propylene sulfide) to obtain protein nanovaccines[38][39][40][41]. These polymer-conjugated protein vaccines elicited stronger immune responses against different antigens, in comparison to antigens alone or antigens delivered with polymeric material in physical mixture form.
2.4 Inorganic-based nanovaccines
Inorganic nanoscale particles can also be associated with vaccine components to elevate immune responses. Metallic NPs, especially gold NPs, have been recognized as promising vaccine carriers due to their relatively high biocompatibility, easily controllable size range, and high surface area. Protein antigens have usually been complexed with gold NPs via electrostatic interactions or metal chelating to obtain protein nanovaccines[42][43][44][45][46]; however, covalent linkages can also be formed when gold nanoparticles are functionalized with particular moieties, such as carboxyl groups, through the reaction between sulfur and gold[47][48]. Antigens conjugated to gold nanoparticles were reported to generate higher IgG titers compared to antigens alone. Non-metallic inorganic NPs, such as mesoporous silica NPs, have also been reported to be efficient carriers for the delivery of protein antigens [49].
3. Future Perspective
Subunit vaccines, especially protein-based vaccines, can induce both cellular and humoral responses with the help of an appropriate adjuvant or delivery system. Once produced as nanostructures, protein-based subunit vaccines can elicit stronger immune responses compared to soluble antigens alone. While it might be easier and more cost-efficient to associate these nanocarriers with antigens via noncovalent interactions, such as physical encapsulation or electrostatic interactions, covalent linkage can improve antigen delivery into the APCs, thereby eliciting a stronger and more specific immune response. With advances in nanotechnology, as well as biological and chemical engineering technologies, it is believed that protein-based subunit nanovaccines will help satisfy the unmet demands of preventing and controlling different infectious diseases in the near future.
References
- Kathryn A. Hanley; The Double-Edged Sword: How Evolution Can Make or Break a Live-Attenuated Virus Vaccine. Evolution: Education and Outreach 2011, 4, 635-643, 10.1007/s12052-011-0365-y.
- Phillip M. Lovalenti; Jeff Anderl; Luisa Yee; Van Nguyen; Behnaz Ghavami; Satoshi Ohtake; Atul Saxena; Thomas Voss; Vu Truong-Le; Stabilization of Live Attenuated Influenza Vaccines by Freeze Drying, Spray Drying, and Foam Drying. Pharmaceutical Research 2016, 33, 1144-1160, 10.1007/s11095-016-1860-1.
- Peter Michael Moyle; Istvan Toth; Modern Subunit Vaccines: Development, Components, and Research Opportunities. ChemMedChem 2013, 8, 360-376, 10.1002/cmdc.201200487.
- Valeria A. Sander; Edwin F. Sánchez López; Luisa Mendoza Morales; Victor A. Ramos Duarte; Mariana G. Corigliano; Marina Clemente; Use of Veterinary Vaccines for Livestock as a Strategy to Control Foodborne Parasitic Diseases. Frontiers in Cellular and Infection Microbiology 2020, 10, 288, 10.3389/fcimb.2020.00288.
- Morgan Brisse; Sophia M. Vrba; Natalie Kirk; Yuying Liang; Hinh Ly; Emerging Concepts and Technologies in Vaccine Development. Frontiers in Immunology 2020, 11, 583077, 10.3389/fimmu.2020.583077.
- Michael James Francis; Recent Advances in Vaccine Technologies. Veterinary Clinics of North America: Small Animal Practice 2017, 48, 231-241, 10.1016/j.cvsm.2017.10.002.
- Lixin Liu; Zhijia Liu; Haolin Chen; Hong Liu; Qiang Gao; Feng Cong; Guangxia Gao; Yongming Chen; Subunit Nanovaccine with Potent Cellular and Mucosal Immunity for COVID-19. ACS Applied Bio Materials 2020, 3, 5633-5638, 10.1021/acsabm.0c00668.
- Matthew D. Shin; Sourabh Shukla; Young Hun Chung; Veronique Beiss; Soo Khim Chan; Oscar A. Ortega-Rivera; David M. Wirth; Angela Chen; Markus Sack; Jonathan K. Pokorski; et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nature Nanotechnology 2020, 15, 646-655, 10.1038/s41565-020-0737-y.
- Mariusz Skwarczynski; Istvan Toth; Peptide-based synthetic vaccines. Chemical Science 2015, 7, 842-854, 10.1039/c5sc03892h.
- Sujin Lee; Minh Trang Nguyen; Recent Advances of Vaccine Adjuvants for Infectious Diseases. Immune Network 2015, 15, 51-57, 10.4110/in.2015.15.2.51.
- Zhi-Biao Wang; Jing Xu; Better Adjuvants for Better Vaccines: Progress in Adjuvant Delivery Systems, Modifications, and Adjuvant–Antigen Codelivery. Vaccines 2020, 8, 128, 10.3390/vaccines8010128.
- Guangzu Zhao; Saranya Chandrudu; Mariusz Skwarczynski; Istvan Toth; The application of self-assembled nanostructures in peptide-based subunit vaccine development. European Polymer Journal 2017, 93, 670-681, 10.1016/j.eurpolymj.2017.02.014.
- Sahra Bashiri; Prashamsa Koirala; Istvan Toth; Mariusz Skwarczynski; Carbohydrate Immune Adjuvants in Subunit Vaccines. Pharmaceutics 2020, 12, 965, 10.3390/pharmaceutics12100965.
- Lantian Lu; Viet Duong; Ahmed Shalash; Mariusz Skwarczynski; Istvan Toth; Chemical Conjugation Strategies for the Development of Protein-Based Subunit Nanovaccines. Vaccines 2021, 9, 563, 10.3390/vaccines9060563.
- Prateek Bhardwaj; Eshant Bhatia; Shivam Sharma; Nadim Ahamad; Rinti Banerjee; Advancements in prophylactic and therapeutic nanovaccines. Acta Biomaterialia 2020, 108, 1-21, 10.1016/j.actbio.2020.03.020.
- Saghi Nooraei; Howra Bahrulolum; Zakieh Sadat Hoseini; Camellia Katalani; Abbas Hajizade; Andrew J. Easton; Gholamreza Ahmadian; Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. Journal of Nanobiotechnology 2021, 19, 1-27, 10.1186/s12951-021-00806-7.
- Nicholas M. Molino; Medea Neek; Jo Anne Tucker; Edward Nelson; Szu-Wen Wang; Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials 2016, 86, 83-91, 10.1016/j.biomaterials.2016.01.056.
- Medea Neek; Jo Anne Tucker; Tae Il Kim; Nicholas M. Molino; Edward L. Nelson; Szu-Wen Wang; Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials 2017, 156, 194-203, 10.1016/j.biomaterials.2017.11.022.
- Nicholas M. Molino; Amanda K. L. Anderson; Edward L. Nelson; Szu-Wen Wang; Biomimetic Protein Nanoparticles Facilitate Enhanced Dendritic Cell Activation and Cross-Presentation. ACS Nano 2013, 7, 9743-9752, 10.1021/nn403085w.
- W. Berger; E. Steiner; Michael Grusch; L. Elbling; M. Micksche; Vaults and the major vault protein: Novel roles in signal pathway regulation and immunity. Experientia 2008, 66, 43-61, 10.1007/s00018-008-8364-z.
- Leonard H. Rome; Valerie Kickhoefer; Development of the Vault Particle as a Platform Technology. ACS Nano 2012, 7, 889-902, 10.1021/nn3052082.
- Cheryl I. Champion; Valerie A. Kickhoefer; Guangchao Liu; Raymond J. Moniz; Amanda S. Freed; Liisa L. Bergmann; Dana Vaccari; Sujna Raval-Fernandes; Ann M. Chan; Leonard H. Rome; et al. A Vault Nanoparticle Vaccine Induces Protective Mucosal Immunity. PLOS ONE 2009, 4, e5409, 10.1371/journal.pone.0005409.
- Upendra Kar; Janina Jiang; Cheryl I. Champion; Sahar Salehi; Minu Srivastava; Sherven Sharma; Shahrooz Rabizadeh; Kayvan Niazi; Valerie Kickhoefer; Leonard Rome; et al. Vault Nanocapsules as Adjuvants Favor Cell-Mediated over Antibody-Mediated Immune Responses following Immunization of Mice. PLOS ONE 2012, 7, e38553, 10.1371/journal.pone.0038553.
- Janina Jiang; Guangchao Liu; Valerie A. Kickhoefer; Leonard H. Rome; Lin-Xi Li; Stephen J. McSorley; Kathleen A. Kelly; A Protective Vaccine against Chlamydia Genital Infection Using Vault Nanoparticles without an Added Adjuvant. Vaccines 2017, 5, 3, 10.3390/vaccines5010003.
- Zhongyan Wang; Yuna Shang; Zhaoqi Tan; Xiaoyan Li; Guoliang Li; Chunhua Ren; Fuqiang Wang; Zhimou Yang; Jianfeng Liu; A supramolecular protein chaperone for vaccine delivery. Theranostics 2020, 10, 657-670, 10.7150/thno.39132.
- Rui Kuai; Lukasz J. Ochyl; Keith S. Bahjat; Anna Schwendeman; Rui Kuai Lukasz J. Ochyl Anna Schwendeman James J. Moon; Designer vaccine nanodiscs for personalized cancer immunotherapy. Nature Materials 2016, 16, 489-496, 10.1038/nmat4822.
- John C. Kraft; Jennifer P. Freeling; Ziyao. Wang; Rodney J.Y. Ho; Emerging Research and Clinical Development Trends of Liposome and Lipid Nanoparticle Drug Delivery Systems. Journal of Pharmaceutical Sciences 2014, 103, 29-52, 10.1002/jps.23773.
- M. Danaei; M. Dehghankhold; S. Ataei; F. Hasanzadeh Davarani; R. Javanmard; A. Dokhani; S. Khorasani; M. R. Mozafari; Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57, 10.3390/pharmaceutics10020057.
- Susan Hua; Maria B. C. De Matos; Josbert M. Metselaar; Gert Storm; Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Frontiers in Pharmacology 2018, 9, 790, 10.3389/fphar.2018.00790.
- Abolfazl Akbarzadeh; Rogaie Rezaei-Sadabady; Soodabeh Davaran; Sang Woo Joo; Nosratollah Zarghami; Younes Hanifehpour; Mohammad Samiei; Mohammad Kouhi; Kazem Nejati-Koshki; Liposome: classification, preparation, and applications. Nanoscale Research Letters 2013, 8, 102-102, 10.1186/1556-276x-8-102.
- Reto A. Schwendener; Liposomes as vaccine delivery systems: a review of the recent advances. Therapeutic Advances in Vaccines 2014, 2, 159-182, 10.1177/2051013614541440.
- Matthias Pauthner; Colin Havenar-Daughton; Devin Sok; Joseph P. Nkolola; RaizA Bastidas; Archana V. Boopathy; Diane G. Carnathan; Abishek Chandrashekar; Kimberly M. Cirelli; Christopher A. Cottrell; et al. Elicitation of Robust Tier 2 Neutralizing Antibody Responses in Nonhuman Primates by HIV Envelope Trimer Immunization Using Optimized Approaches. Immunity 2017, 46, 1073-1088.e6, 10.1016/j.immuni.2017.05.007.
- Shridhar Bale; Geraldine Goebrecht; Armando Stano; Richard Wilson; Takayuki Ota; Karen Tran; Jidnyasa Ingale; Michael B. Zwick; Richard T. Wyatt; Covalent Linkage of HIV-1 Trimers to Synthetic Liposomes Elicits Improved B Cell and Antibody Responses. Journal of Virology 2017, 91, e00443-17, 10.1128/jvi.00443-17.
- Khairunnisa Abdul Ghaffar; Nirmal Marasini; Ashwini Kumar Giddam; Michael R. Batzloff; Michael F. Good; Mariusz Skwarczynski; Istvan Toth; Liposome-based intranasal delivery of lipopeptide vaccine candidates against group A streptococcus. Acta Biomaterialia 2016, 41, 161-168, 10.1016/j.actbio.2016.04.012.
- Natassa Pippa; Maria Gazouli; Stergios Pispas; Recent Advances and Future Perspectives in Polymer-Based Nanovaccines. Vaccines 2021, 9, 558, 10.3390/vaccines9060558.
- Reshma J. Nevagi; Mariusz Skwarczynski; Istvan Toth; Polymers for subunit vaccine delivery. European Polymer Journal 2019, 114, 397-410, 10.1016/j.eurpolymj.2019.03.009.
- Lili Zhao; Mariusz Skwarczynski; Istvan Toth; Polyelectrolyte-Based Platforms for the Delivery of Peptides and Proteins. ACS Biomaterials Science & Engineering 2019, 5, 4937-4950, 10.1021/acsbiomaterials.9b01135.
- Hiroko Toyota; Noriko Yanase; Takayuki Yoshimoto; Mitsunori Harada; Yasuki Kato; Junichiro Mizuguchi; Vaccination with OVA-bound nanoparticles encapsulating IL-7 inhibits the growth of OVA-expressing E.G7 tumor cells in vivo. Oncology Reports 2014, 33, 292-296, 10.3892/or.2014.3603.
- Qingfeng Liu; Xiaoyao Zheng; Chi Zhang; Xiayan Shao; Xi Zhang; Qizhi Zhang; Xinguo Jiang; Conjugating influenza a (H1N1) antigen to n-trimethylaminoethylmethacrylate chitosan nanoparticles improves the immunogenicity of the antigen after nasal administration. Journal of Medical Virology 2015, 87, 1807-1815, 10.1002/jmv.24253.
- Kirsty L. Wilson; Dodie Pouniotis; Jennifer Hanley; Sue D. Xiang; Charles Ma; Ross L. Coppel; Magdalena Plebanski; A Synthetic Nanoparticle Based Vaccine Approach Targeting MSP4/5 Is Immunogenic and Induces Moderate Protection Against Murine Blood-Stage Malaria. Frontiers in Immunology 2019, 10, 331, 10.3389/fimmu.2019.00331.
- Armando Stano; Evan Scott; Karen Y. Dane; Melody A. Swartz; Jeffrey A. Hubbell; Tunable T cell immunity towards a protein antigen using polymersomes vs. solid-core nanoparticles. Biomaterials 2013, 34, 4339-4346, 10.1016/j.biomaterials.2013.02.024.
- Kenichi Niikura; Tatsuya Matsunaga; Tadaki Suzuki; Shintaro Kobayashi; Hiroki Yamaguchi; Yasuko Orba; Akira Kawaguchi; Hideki Hasegawa; Kiichi Kajino; Takafumi Ninomiya; et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo. ACS Nano 2013, 7, 3926-3938, 10.1021/nn3057005.
- Chao Wang; Wandi Zhu; Yuan Luo; Bao-Zhong Wang; Gold nanoparticles conjugating recombinant influenza hemagglutinin trimers and flagellin enhanced mucosal cellular immunity. Nanomedicine: Nanotechnology, Biology and Medicine 2018, 14, 1349-1360, 10.1016/j.nano.2018.03.007.
- Chao Wang; Wandi Zhu; Bao-Zhong Wang; Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro. International Journal of Nanomedicine 2017, ume 12, 4747-4762, 10.2147/ijn.s137222.
- John W. Stone; Natalie J. Thornburg; David L. Blum; Sam J. Kuhn; David W. Wright; James E Crowe Jr; James E. Crowe; Gold nanorod vaccine for respiratory syncytial virus. Nanotechnology 2013, 24, 295102, 10.1088/0957-4484/24/29/295102.
- Hanako Sekimukai; Naoko Iwata‐Yoshikawa; Shuetsu Fukushi; Hideki Tani; Michiyo Kataoka; Tadaki Suzuki; Hideki Hasegawa; Kenichi Niikura; Katsuhiko Arai; Noriyo Nagata; et al. Gold nanoparticle‐adjuvanted S protein induces a strong antigen‐specific IgG response against severe acute respiratory syndrome‐related coronavirus infection, but fails to induce protective antibodies and limit eosinophilic infiltration in lungs. Microbiology and Immunology 2019, 64, 33-51, 10.1111/1348-0421.12754.
- A.E. Gregory; E.D. Williamson; J.L. Prior; W.A. Butcher; I.J. Thompson; Andrew Shaw; R.W. Titball; Conjugation of Y. pestis F1-antigen to gold nanoparticles improves immunogenicity. Vaccine 2012, 30, 6777-6782, 10.1016/j.vaccine.2012.09.021.
- Lev A. Dykman; Gold nanoparticles for preparation of antibodies and vaccines against infectious diseases. Expert Review of Vaccines 2020, 19, 465-477, 10.1080/14760584.2020.1758070.
- Stefanie Thalhauser; David Peterhoff; Ralf Wagner; Miriam Breunig; Presentation of HIV-1 Envelope Trimers on the Surface of Silica Nanoparticles. Journal of Pharmaceutical Sciences 2019, 109, 911-921, 10.1016/j.xphs.2019.10.059.
