Currently, carbon nanoparticles play a large role as carriers of various types of drugs, and also have applications in other fields of medicine, e.g., in tissue engineering, where they are used to reconstruct bone tissue. They also contribute to the early detection of cancer cells, and can act as markers in imaging diagnostics. Their antibacterial and anti-inflammatory properties are also known. This feature is particularly important in dental implantology, where various types of bacterial infections and implant rejection often occur. The search for newer and more effective treatments may lead to future use of nanoparticles on a large scale.
- fullerenes
- nanotubes
- nanodiamonds
- cancer
- theranostics
- bone reconstruction
1. Introduction
Today, nanotechnology is one of the most dynamically developing sciences. Increasingly, nanotechnological achievements are used in the biological and medical fields. This application is possible due to the production of nanoparticles, where the size does not exceed 100 nm [1][2][3]. Considering their origin, nanoparticles can be divided into: Natural and designed, and considering their structure, into spherical, fibrous and layered [4]. Natural nanoparticles are formed in nature as a result of, for example, the erosion of geological materials, as well as decomposition of biological materials, mainly plant residues. They can also be produced as a result of combustion of fuel products [5][6][7]. Designed nanoparticles are manufactured by the nanotechnology industry. They are characterized by variable physical and chemical properties, a specific size and various modifications of their surface. Due to these modifications, they demonstrate very good adsorption and absorption properties, and are also able to aggregate. All these features of nanoparticles make them useful in many fields, e.g., nanomedicine, nanopharmacology and nanooncology. All these properties are met by carbon nanoparticles.
2. Transport of Carbon Nanoparticles into Cells
2.1. Transport of Nanoparticles to Cancer Cells
As carbon nanoparticles are a promising material to be used in medicine, mainly as drug carriers, in order to recognize and destroy cancer cells, it is important to understand how specific agents are delivered to tumor areas. Most nanoparticles are able to transport drugs to tumor cells in a passive manner, taking advantage of selectively increased permeability and retention of tumor vessels [8], or by active transport through endocytic pathways. Molecules coated with various ligands bind to cell receptors and penetrate inside a cell by endocytosis, thus, delivering a higher concentration of a drug to the inside of a cancer cell, without causing greater cytotoxicity to normal cells [9]. Possible ways of nanoparticles transport into cancer cells are shown in Scheme 1 . All routes of entry are preferred in cancer cells compared to healthy cells due to the differences in the biochemistry of these cells.
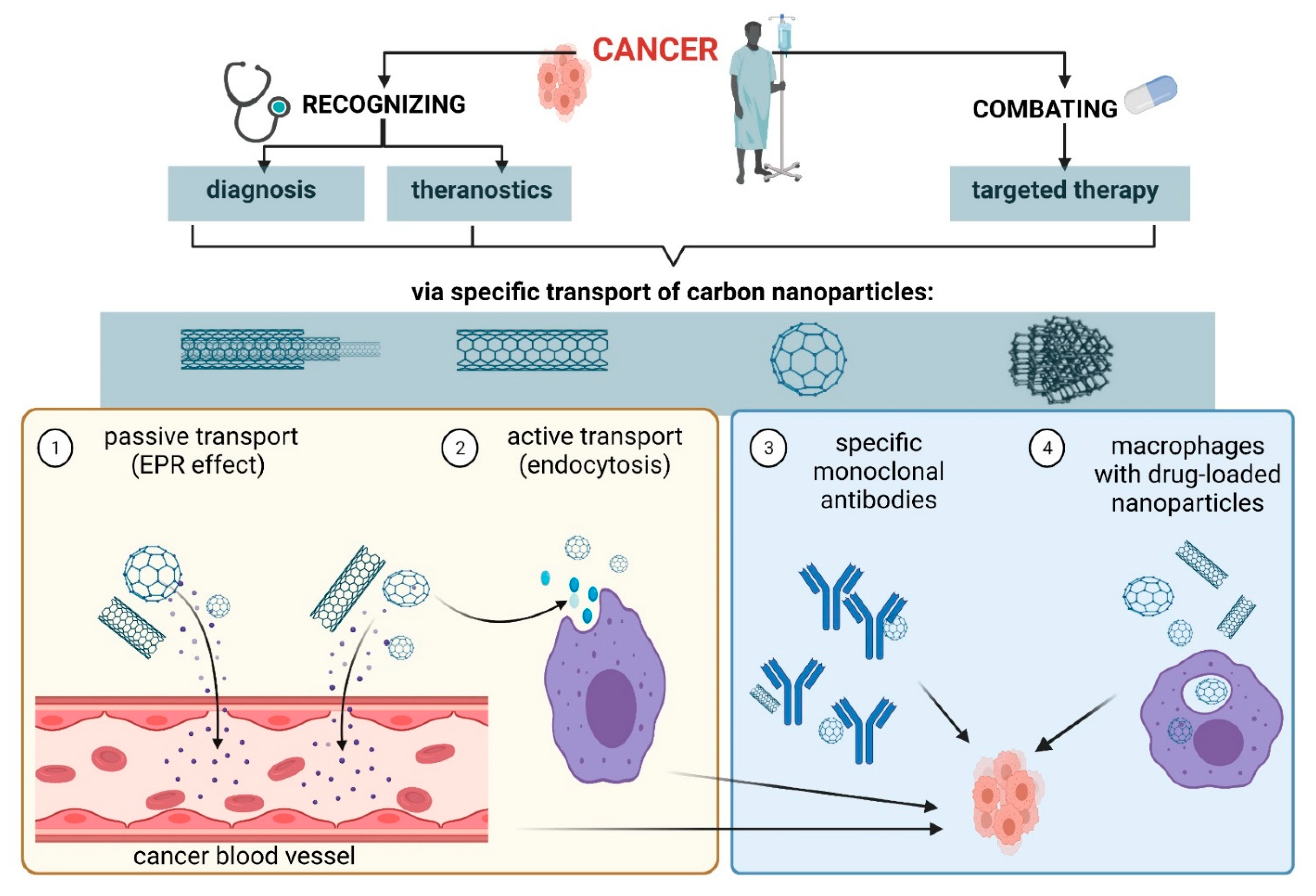 Scheme 1. Transport of carbon nanoparticles into cancer cells. Created with BioRender.com accessed on 13 June 2021.
Scheme 1. Transport of carbon nanoparticles into cancer cells. Created with BioRender.com accessed on 13 June 2021.
The needle-like shape of the NTs allows them to cross the cell membrane as a result of endocytosis and enter cancer cells to deliver the attached drug [10]. Efficacy of ND transport by the endocytic route was investigated using A549 human lung cancer cells and the HFL1 human non-cancerous lung fibroblast cell line, and Beas-2b human bronchial epithelial cells. It was shown that ND penetrated cancer cells to a much greater extent than normal cells, by clathrin-dependent endocytosis [11][12]. Research by Solarska-Ściuk et al. [11] showed that NDs entered the cytoplasm of cells by both clathrin-dependent endocytosis and micropinocytosis, without disturbing the function of endothelial and epithelial cells at concentrations of up to 50 µg/mL after 24 h of incubation. The used cell lines of both human and animal origins allow us to predict the transport of nanoparticles through cell membranes in vivo, and to conduct research on the bioavailability of various drugs. Therefore, they are becoming increasingly used as useful tools for the assessment of active substances.
The endocytic pathway is essential not only for the transport of drugs but also of nutrients that cross the blood-brain barrier [13]. Research has shown that multi-walled carbon nanotubes (MWCNTs) crossed the blood-brain barrier (BBB). In the work of Gonzalez-Carter et al. [14], functionalized anionic, cationic and non-ionic MWCNTs were examined, in order to investigate the cellular uptake across the blood-brain barrier. the results showed that a large proportion of the cationic and non-ionic, but not anionic, MWCNTs remained in the brain’s endothelial cell membrane. The uptake of MWCNTs by brain endothelial cells is low (<1.5%) and does not correlate with BBB translocation. Anionic MWCNTs have the highest transport levels in the in vitro human BBB model compared to non-ionic or cationic nanotubes [14]. This is confirmed by Moscariello et al. [15] who showed that fluorescent NDs surrounded by a biopolymer coating based on human serum albumin (dcHSA-PEG) were taken up by target brain cells. The use of dcHSA-ND confirms the ability of complexes/conjugates to cross the BBB in a mouse model. Observation of dcHSA-ND is possible at the single cell level and reveals its in vivo uptake into neurons and astrocytes. This study shows NDs penetration into the brain via a BBB transport mechanism [15].
Recently, the use of macrophages as cells for capturing nanoparticles has been particularly interesting [16]. In response to the Abraxan-induced apoptosis of MDA-MB-435 tumor cells in mice, infiltration of macrophages in the tumor region was observed. Those macrophages captured drug-loaded nanoparticles and targeted them to cancer cells. Macrophages can quickly and directionally migrate to pathological sites where specific chemokines are secreted, which allows them to serve as targeted drug delivery vehicles [17][18]. There are two types of macrophages that target M1 and M2 tumor cells, called Tumor Associated Macrophages (TAMs). TAMs are mainly induced by IL-6 and IL-10. They protect cancer stem cells, paving the way for metastasis, and weaken protective adaptive immunity [19]. During tumor initiation, TAMs exhibit properties characteristic of M1 macrophages, which condition the activation of pro-inflammatory intracellular pathways dependent on transcription factors (NF-KB). In turn, with the development of cancer, these cells transform into M2 macrophages, that inhibit signaling pathways determining expression of pro-inflammatory cytokines. TAMs also inhibit T cell activity thereby promoting tumor progression [17][19][20][21]. In studies of skin cancer and lung metastases in experimental mice, it was found that triterpenoid compounds (e.g., oleanolic acid) reduced the transcription factor STAT3 (Signal Transducers and Activators of Transcription) and inhibit macrophage polarization towards M2. These compounds also increased sensitivity of cancer cells to the action of anti-cancer drugs: Adriamycin and cisplatin [20].
2.2. Passing the Blood-Brain Barrier
2.3. Macrophages in Transport of Nanoparticles
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/ijms22158341
References
- Bera, A.; Belhaj, H. Application of nanotechnology by means of nanoparticles and nanodispersions in oil recovery—A comprehensive review. J. Nat. Gas. Sci. Eng. 2016, 34, 1284–1309.
- Mnyusiwalla, A.; Daar, A.S.; Singer, P.A. Mind the gap: Science and ethics in nanotechnology. Nanotechnology 2003, 14, R9–R13.
- Attota, R.K.; Liu, E.C. Volume determination of irregularly-shaped quasi-spherical nanoparticles. Anal. Bioanal. Chem. 2016, 408, 7897–7903.
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931.
- Slezakova, K.; Morais, S.; Carmo Pereir, M.D. Atmospheric Nanoparticles and Their Impacts on Public Health; InTech: London, UK, 2013.
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22.
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein. J. Nanotechnol 2018, 9, 1050–1074.
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284.
- Prabhakar, N.; Khan, M.H.; Peurla, M.; Chang, H.-C.; Hänninen, P.E.; Rosenholm, J.M. Intracellular Trafficking of Fluorescent Nanodiamonds and Regulation of Their Cellular Toxicity. ACS Omega 2017, 2, 2689–2693.
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79.
- Solarska-Ściuk, K.; Gajewska, A.; Glińska, S.; Studzian, M.; Michlewska, S.; Balcerzak, Ł.; Skolimowski, J.; Kolago, B.; Bartosz, G. Intracellular transport of nanodiamond particles in human endothelial and epithelial cells. Chem. Biol. Interact. 2014, 219, 90–100.
- Perevedentseva, E.; Hong, S.F.; Huang, K.J.; Chiang, I.T.; Lee, C.Y.; Tseng, Y.T.; Cheng, C.L. Nanodiamond internalization in cells and the cell uptake mechanism. J. Nanoparticle Res. 2013, 15, 1834.
- Sharma, G.; Sharma, A.R.; Lee, S.-S.; Bhattacharya, M.; Nam, J.-S.; Chakraborty, C. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 2019, 559, 360–372.
- Gonzalez-Carter, D.; Goode, A.E.; Kiryushko, D.; Masuda, S.; Hu, S.; Lopes-Rodrigues, R.; Dexter, D.T.; Shaffer, M.S.P.; Porter, A.E. Quantification of blood–brain barrier transport and neuronal toxicity of unlabelled multiwalled carbon nanotubes as a function of surface charge. Nanoscale 2019, 11, 22054–22069.
- Moscariello, P.; Raabe, M.; Liu, W.; Bernhardt, S.; Qi, H.; Kaiser, U.; Wu, Y.; Weil, T.; Luhmann, H.J.; Hedrich, J. Unraveling In Vivo Brain Transport of Protein-Coated Fluorescent Nanodiamonds. Small 2019, 15, e1902992.
- Moura, R.P.; Almeida, A.; Sarmento, B. The role of non-endothelial cells on the penetration of nanoparticles through the blood brain barrier. Prog. Neurobiol. 2017, 159, 39–49.
- Cao, Q.; Yan, X.; Chen, K.; Huang, Q.; Melancon, M.P.; Lopez, G.; Cheng, Z.; Li, C. Macrophages as a potential tumor-microenvironment target for noninvasive imaging of early response to anticancer therapy. Biomaterials 2018, 152, 63–76.
- Pang, L.; Qin, J.; Han, L.; Zhao, W.; Liang, J.; Xie, Z.; Yang, P.; Wang, J. Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget 2016, 7, 37081–37091.
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416.
- Fujiwara, Y.; Takeya, M.; Komohara, Y. A novel strategy for inducing the antitumor effects of triterpenoid compounds: Blocking the protumoral functions of tumor-associated macrophages via STAT3 inhibition. Biomed. Res. Int. 2014, 2014, 348539.
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529.
