Trifarotene is a new fourth-generation retinoid with a selective action on RAR-γ.
- acne
- congenital ichthyosis
- T Cell lymphoma
- Retinoids
- Trifarotene
1. Introduction
Vitamin A (or retinol, a diterpene), a cardinal micronutrient in human metabolism, is a lipophilic molecule composed by isoprene units. As an isoprenoid, it is characterized by a hydrocarbon chain containing an ending hydroxyl. The term “retinoid” concerns both natural and synthetic analogues of vitamin A. In synthetic analogues, such as etretinate, acitretin, or tazarotene, a benzene ring substitutes the cyclohexane. According to the International Union of Pure and Applied Chemistry and the International Union of Biochemistry and Molecular Biology, retinoids are characterized by four isoprene units with a head-to-tail structure [1].
It is known that vitamin A and its synthetic analogues have a crucial role in modulating some skin functions; in particular, they regulate epidermal keratinization, differentiation, maturation, and proliferation [2]. Due to all these effects, retinoids are largely used in dermato-oncology, both in treatment and chemo-prevention (non-melanoma skin cancers, primary cutaneous T-cell lymphomas), and even in the treatment of cutaneous inflammatory diseases (acne vulgaris, rosacea, melasma, post-inflammatory hyperpigmentation, mycosis) and hyperproliferative conditions (ichtyosis, psoriasis, pityriasis rubra pilaris) [2]. Moreover, they play a central role in protecting the skin from free radicals damage, as shown by their use also in photoaging. The aim of this review is to highlight the current clinical application (Figure 1) of trifarotene and future perspectives in dermatology.
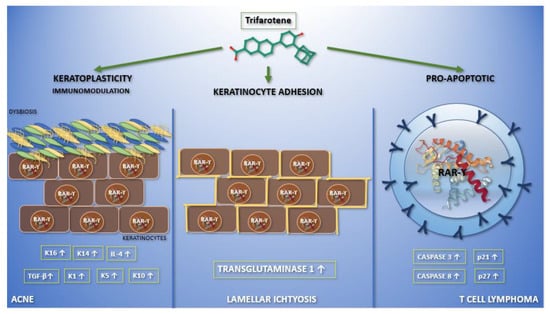
Figure 1. Graphic abstract representing the current applications of trifarotene in dermatological clinical trials and molecular pathways. In the left section, the action of trifarotene in acne is reported. It performs both immunoregulatory—leading to an increase in the expression of transforming growth factor-β and interleukin-4—and keratoplastic functions, increasing the expression of keratins K1, K5, K10, K14, and K16. Moreover, it seems that trifarotene, like other retinoids, may have a role in modulating skin microbiota. Finally, trifarotene weakens hemidesmosomes, interfering with cell adhesion. The migration of keratinocytes, caused by the drug, mediates its comedolytic property. The importance of trifarotene in lamellar ichthyosis has been reported in the central section. Trifarotene, by means of RAR-γ, causes an increased expression of transglutaminase 1, promoting keratinocyte cohesion. The rationale for the use of trifarotene in cutaneous T cell lymphomas has been reported in the right section. It seems to promote apoptosis and differentiation, upregulating caspases 3 and 8, p21 and p27.
2. Trifarotene Properties and Current Applications in Dermatology
2.1. RAR-γ Selectivity
RAR-γ selective retinoid derivatives such as trifarotene, are being investigated as topical agents, which are expected to offer a more favorable clinical profile compared to the dual RARβ/γ drugs currently used in the clinical practice. Trifarotene is a potent and selective agonist of RAR-γ, with significantly less activity on RAR-β and RAR-α (16- and 65-fold lower, respectively), and has no activity on RXRs [3]. Although tazarotene has high affinity for all retinoic acid receptor isoforms, its affinity is roughly 5–8 times higher for the β isoform [4]. Adapalene is selective for the β and γ RARs over the α isoform [5]. Selectivity is a key feature in trifarotene, which allows the action on the keratinocytes, as a primary target, and reduces systemic adverse effects (Table 1). We know, from the Human Protein Atlas project, that RAR-γ m-RNA has the maximum expression in the skin (mean reads per kb per million reads placed 47.135 ± 3.294, compared to RAR-β 0.272 ± 0.138, count 3,210,032 for RAR-γ vs. 21,114 for RAR-β; Figure S1) [6], sustaining the rational use of a topical RAR-γ agonist.
Table 1. The table shows the different drugs capable of interacting with the different RAR isoforms. Of all the drugs reported, only trifarotene and palovarotene are selective for RAR-γ. Currently, palovarotene is used in the treatment of progressive ossifying fibrodysplasia, while trifarotene is used in lamellar ichthyosis and acne. The importance of receptor selectivity is confirmed by the reduction of adverse effects due to the action on the other receptors.
| Receptor | RAR-α | RAR-β | RAR-γ | |
|---|---|---|---|---|
| Tissue Expression | Lung, Spleen, Gallbladder | Placenta, Prostate, Urinary Bladder, Kidney, Heart | Skin | |
| Drug | Tazarotene | ☑ | ☑ | ☑ |
| Tretinoin | ☑ | ☑ | ☑ | |
| Trifarotene | X | X | ☑ | |
| Adapalene | X | ☑ | ☑ | |
| Alitetrinoin | ☑ | ☑ | ☑ | |
| Tamibarotene | ☑ | X | X | |
| Palovarotene | X | X | ☑ | |
2.2. Trifarotene Safety and Tolerability
Trifarotene is metabolized in vitro by CYP2C9, CYP3A4, CYP2C8, and, to a lesser extent, by CYP2B6, and excreted in the feces [7]. Systemic exposures in mice, following both topical and oral administration, were up to 1642 times higher than those seen in humans at the maximal recommended human dose, and these systemic concentrations did not result in observed carcinogenicity. Trifarotene does not seem to carry any risk of carcinogenesis when used at standard doses [8][9]. Data regarding overdosage of trifarotene are not available. Patients exposed to photosensitising agents may have an increased risk of a phototoxic skin reaction, especially severe sunburn, during the use of aminolevulinic acid [10]. Concomitant use of retinoids and keratolytic or topical astringents may result in excessive irritation and/or drying, and patients may experience erythema, scaling, dryness, and stinging/burning [8][9]. Despite these local adverse effects and photosensitization, trifarotene is safer than other retinoids due to its hepatic instability and degradation. Current studies confirm that trifarotene 50 µg/g cream is systemically well tolerated and safe when applied under maximized conditions in adults and pediatric acne patients, including patients with severe acne [9]. Considering that trifarotene belongs to the class of retinoids and is intended for use even in women in their fertile age, further studies are needed to exclude any potential teratogenic effect. Currently, clinical pharmacological data demonstrate that trifarotene 50 µg/g cream—the to-be-marketed formulation—generates low systemic absorption when applied daily under maximal use conditions [9]. Furthermore, due to its RAR-γ selectivity, it could be hypothesized that trifarotene is safer than other retinoids in pregnancy as the placenta presents a lower expression of RAR-γ (2.739 ± 0.399), with consequent minor absorption of the drug compared to other topical retinoids [6].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines9030237
References
- Nomenclature of retinoids. Recommendations. Eur. J. Biochem. 1982, 129, 1–5.
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Ventura, A.; Dika, E.; Gaziano, R.; Dattola, A.; Candi, E.; Bianchi, L. Predictive role of vitamin A serum concentration in psoriatic patients treated with IL-17 inhibitors to prevent skin and systemic fungal infections. J. Pharmacol. Sci. 2020, 144, 52–56.
- Aubert, J.; Piwnica, D.; Bertino, B.; Blanchet-Réthoré, S.; Carlavan, I.; Déret, S.; Dreno, B.; Gamboa, B.; Jomard, A.; Luzy, A.; et al. Nonclinical and human pharmacology of the potent and selective topical retinoic acid receptor-γ agonist trifarotene. Br. J. Dermatol. 2018, 179, 442–456.
- Charton, J.; Deprez-Poulain, R.; Hennuyer, N.; Tailleux, A.; Staels, B.; Deprez, B. Novel non-carboxylic acid retinoids: 1,2,4-Oxadiazol-5-one derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 489–492.
- Charpentier, B.; Bernardon, J.-M.; Eustache, J.; Millois, C.; Martin, B.; Michel, S.; Shroot, B. Synthesis, Structure-Affinity Relationships, and Biological Activities of Ligands Binding to Retinoic Acid Receptor Subtypes. J. Med. Chem. 1995, 38, 4993–5006.
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-specific Expression by Genome-wide Integration of Transcriptomics and Antibody-based Proteomics. Mol. Cell. Proteom. 2014, 13, 397–406.
- Thoreau, E.; Arlabosse, J.-M.; Bouix-Peter, C.; Chambon, S.; Chantalat, L.; Daver, S.; Dumais, L.; Duvert, G.; Feret, A.; Ouvry, G.; et al. Structure-based design of Trifarotene (CD5789), a potent and selective RARγ agonist for the treatment of acne. Bioorg. Med. Chem. Lett. 2018, 28, 1736–1741.
- Maynepharma. Available online: (accessed on 6 August 2020).
- Ms, N.W.; Benkali, K.; Sáenz, A.A.; Poncet, M.; Graeber, M. Clinical Pharmacology and Safety of Trifarotene, a First-in-Class RARγ-Selective Topical Retinoid. J. Clin. Pharmacol. 2020, 60, 660–668.
- Drugs.com. Gleolan (Aminolevulinic Acid) Drug Interactions from Drugs.com; c1996–2018 [Updated: 20 November 2018]. Available online: (accessed on 6 August 2020).
