Dermal wound healing describes the progressive repair and recalcitrant mechanism of damaged skin, and eventually, reformatting and reshaping the skin. Many probiotics, nutritional supplements, metal nanoparticles, composites, skin constructs, polymers, and so forth have been associated with the improved healing process of wounds. The exact mechanism of material-cellular interaction is a point of immense importance, particularly in pathological conditions such as diabetes. Bioengineered alternative agents will likely continue to dominate the outpatient and perioperative management of chronic, recalcitrant wounds as new products continue to cut costs and improve the wound healing process.
- dermal wound healing
- nanoceuticals
- metal nanoparticles
- bioengineered alternatives
1. Conventional Therapies Implemented for Healing
1.1. Skin Grafting Techniques
Tissue grafting has been explored for a long time now, with the initial use of autografts going back as far as the 6th century. Skin grafts come into play when the tissue loss or injury is chronic. The graft thickness could either be split-thickness or full-thickness skin grafts [1]. Typically, split-thickness grafts use the epidermis and the papillary dermis of healthy adult skin for repair [1]. Split-thickness grating is known to be the gold standard for a variety of cutaneous wounds, but it comes with certain limitations. Split-thickness procedures fail to repair if the skin loss is more than 1/3rd of the total area of body skin [1]. While meshing can increase the graft sites’ surface area, balancing the meshing ratio, which ideally should be no more than 3:1 (graft: wound area), is hard as it is prone to contracting during repair [2]. Post-grafting symptoms of ache, redness, and inflammation are also observed in such skin grafts. Contrary to split-thickness grafts, full-thickness grafts use both the epidermal and complete dermal layers and are advantageous in repairing soft tissue defects. A full-thickness skin graft can handle chronic injuries well, with less skin shrinking and more aesthetically natural-looking post-healing, unlike split-thickness ones [3]. Full-thickness grafts, however, need a fully vascularized bed for grafting and are affected by donor skin unavailability [4]. Lately, autologous skin graft efficiency has been improved by combining it with scaffolds, gels, therapeutic agents, etc., to accomplish massive full-thickness injuries [3]. Allotransplantation or homografts obtained from genetically dissimilar members of the same species are often beneficial in traumatic wounds, where a temporary graft covering alleviates the recipient’s wound bed until autografting is done [3]. Homografts are immediately available, increase donor supply, and extended storage before use, thus giving them an upper hand in the grafting method. Regrettably, allotransplants are often subjected to viral contaminations such as human immunodeficiency virus, cytomegalovirus, and hepatitis [4]. They might induce strong recipient inflammatory immune reactions with the interference of T and B lymphocytes to ultimately reject the homograft [4]. Recent experiments have shown that implementing mixed chimeric molecules with the donor’s bone marrow could subdue recipient graft rejection in clinical therapies such as the in vitro assay on RA-iTreg cells (retinoic acid), which exhibits immunosuppressive T-cell proliferative activity and also prevents T-cell cytokine activity in mice models [5]. On the other hand, xenotransplants are obtained from heterologous species, with the most frequently used being porcine xenografts, which are ready for use but can cause secondary infections from other dissimilar species. Usually used with burn wounds where <25% of the total skin area is affected, xenografts reduce the implementation of surgical excisions and save time [6].
1.2. Wound Dressings
A dressing is considered ideal if it confers complete wound shielding, eliminates excess exudate, possesses antimicrobial efficacy, maintains a balance between optimum hydration and oxygen, is easy to handle, and has non-anaphylactic properties [7]. Most of the dressings have problems of frequent changing, contamination from the wound fluid, imbalance of wound moisture, difficulty to remove post-application, and insufficient antimicrobial protection. These problems were overcome by using cotton and polymeric bandages to treat dry wounds and those with mild exudation, like the nonocclusive dressing Xeroform ™, made up of a petroleum-based fine mesh gauze with 3% of bismuth tribromophenate, which was used for treating preliminary exudating wounds. Fabric-based non-allergic dressings saturated with paraffin and olive oil, such as Bactigras, Jelonet, Paratulle, etc., were commercially used non-adherent and gamma-sterilized dressings suitable for a superficial clean wound. However, these traditional dressings failed to provide an occlusive hydrated wound healing environment, which paved the way for modern alternatives, viz., contemporary formulated dressings applied directly onto wounds in the form of semi-solid creams, ointments, lotions, and liniments, as well as smart gels, thin self-adherent bioactive incorporated films, etc. The contemporary cotton gauge dressings incorporate chitosan-silver-zinc oxide nanocomposites for efficient moisture retention and antibacterial efficiency [7][8]. During the late 20th century, human amniotic membranes were used for exudate and fluid-laden burn wound dressings. These, as a non-cellular medium for adherence to mesenchymal stem cells, served as a vital platform for skin equivalent development [9]. Although such dressings were advantageous in providing temporary pain relief, balancing the optimum hydration in wounds, and were time- and cost-effective, the chance of infection spread was high [9]. Among polysaccharide dressings, chitosan and chitin are the most explored ones for clinical therapeutics because of their nontoxicity, biocompatibility, high durability, antibacterial efficiency, and suitability to be applied onto open wounds [7]. Their limitations include low tensile strength and elasticity. Algal extract-impregnated dressings have good absorbency, are hemostatic, and antimicrobial; thus, they are useful in exuding wounds [10]. The use of chitosan-alginate amalgamated dressings could improve the mechanical strength and stabilize the dressing [10]. Hyaluronic acid, a linear polysaccharide incorporated into dressings, is compatible with burn, chronic, and surgical wounds [8]. It gives structural sustenance to enable nutrient diffusion, clears wound debris by their interaction with the CD44 molecules, and balances hyperhydration during new tissue regeneration [8]. Hyaluronic acid dressings activate keratinocytes to migrate and proliferate wound sites for ready repair [11]. However, they are highly dissolvable and have less residence time in vivo. Microbial cellulose biosynthesized from Komagataeibacter xylinus, using diverse carbon and nitrogen sources, can precisely be made into dressings for the prophylaxis of extremely chronic injuries that require recurrent dressing changes [7][8]. Unlike other phytocelluloses, microbial ones show substantial pliability, strength, biocompatibility, and good absorbency, but their antimicrobial action limits their medical applications. In addition, they could be improved to a certain extent by the incorporation of nanoparticles like zinc-oxide nanoparticles. Hydrocolloid-based dressings are occlusive dressings for pressure ulcers [12]. They maintain an optimum water and oxygen balance within the wounds, but fail to hold a large amount of exudate. Their frequent changing is necessary to evade the maceration of tissues [12]. Foam dressings are bilaminar structurally with a hydrophilic end with moderate exudate absorbency for wounds with exposed bone. Foam dressings can be rightly called the new substitute to conventional dressings to treat venous pressure ulcers in preventing hospital-acquired pressure ulcers in critically ill individuals. However, they do not yet have much adherence to wound bed and hence are not indorsed for heavy exudative wounds [13]. Adhesive transparent film dressings are suitably conglomerated with hydrogels that allow for optimum wound hydration, maintain skin integrity, and easily monitor the wounds [8]. Research to expand their antimicrobial effectiveness has led to their combination with chlorhexidine, which displays high adherence and declines catheter-related infection to improve vascularization.
1.3. Natural, Phytochemical, and Antiseptic Therapies
Natural and plant-based products have been the traditional ancestral therapies used in skin wound care and management before the rise of pharmaceutical and clinical alternatives. For centuries, these products, because of their potent antimicrobial, anti-inflammatory, anti-analgesic, and cell-stimulating characteristics, were implemented as traditional medicine for acute and chronic wounds. Owing to the existing incidence of diabetes and severe cardiac and vascular implications, chronic wound interventions seek significant attention, making the use of natural therapies for healing applications of specific interest. Natural amalgams encompass a widespread assortment of substances, antioxidants, phenols, terpenes, flavones, and many more organic and inorganic constituents that act as specific targets in the healing process [15]. These constituents have been clinically tested for their efficiency through in vitro and in vivo models. Since wound repair is a complex cascade of biochemical events, it is of utmost importance to stimulate a reparation process without microbial infections. Hence, traditional therapeutic agents and plant-based natural products have shown exemplary outcomes. Further scientific investigation on the progress of various extraction and purification methods, their precise mechanism of action, safety, and quality control assessments, etc., is obligatory. Traditional therapies are cost complaint and beneficial for primary wound care and management. Still, inconsistency in their batch-to-batch results, sudden immunologic reactions, and adverse after-effects can restrict their implication in multidisciplinary wound management [15].
In antimicrobial wound management, topical antiseptics form the first line of a cure for primary wound infection and reduce the microbial bioburden. Antiseptics permeate into wound biofilms, curb bacteria’s growth, and target a broad spectrum of microbial communities, unlike selectively specific antibiotic medications. The most frequently used antiseptics are povidone-iodine, chlorhexidine, alcohol, acetate, hydrogen peroxide, boric acid, silver nitrate, and silver sulfadiazine [16]. Ethanol, isopropanol, and n-propanol are the most suitable agents for surface decontamination and skin asepsis. Their antibactericidal spectrum targets both Gram-positive and Gram-negative bacteria. Another antiseptic used for centuries is iodine and its compounds, which act as a topical agent for preoperative skin preparations [17]. Cadexomer iodine, a hydrophilic modified-starch polymer bead, has been explored for the sustained and controlled release for treating wound exudates [18]. Chlorhexidine, belonging to the class of biguanide, has been extensively investigated as a biocide antiseptic in dermal and oral products either as a 0.05% dilution for wound cleansing or a 4% solution for surgical skin preparation and hand scrub [19]. Polyhexanide biguanide hydrogels have been explored for their cytotoxicity against methicillin-resistant Staphylococcus aureus on dermal wounds and their ability to clinically eradicate the infection. Bisphenols, viz., triclosan and hexachlorophene, are skin-compatible antiseptics effective against Gram-positives. Similarly, silver compounds in the form of solutions, creams, and ointments or nanocrystalline silver have long been used as antimicrobial agents. Their mechanical action is either through cell membrane lysis, the disruption of cellular protein and electron transport chains, or DNA levels by blocking transcription initiation [20]. The expediency of antiseptics on the skin surface, though they are fairly well-established and used, and their utilization as a prophylactic anti-infective agent for open wounds, remains a debatable and unexplored area in the present time. It is relevant to state that a combined traditional and modern therapy approach can target repair faster with negligible side effects, such as silver-impregnated nanofibers, aloe vera extract-embedded alginate hydrogels, propolis wound dressings, honey-based post-operative bandages, etc., could likely expand modern medicine.
1.4. Mechanical Adjuncts and Physical Agents
Despite several attempts to equilibrate the cellular, biomolecular events during wound repair and preserve an optimal hydrated healing environment, there are times when wounds become chronic non-healing sites. A series of mechanical adjuncts and physical agents in use does contribute to such wound reparation processes and provide constructive and adjunctive functions. Hydrotherapy, ultraviolet C radiation (UV-C), vacuum-assisted closure, hyperbaric oxygen, and electrical stimulation are a few worth naming [21]. Hydrotherapy, being one of the oldest adjuvant therapies, is effective for burn wounds where a continuous rotation of water and air eliminates debris and toxic components and dilutes microbial colonization [22]. Hydrotherapy is advantageous for individuals with venous stasis dermatitis, pyoderma gangrenosum, peripheral artery disease, teeth lacerations, and rarely, diabetes mellitus acute wounds. This method effectively upholds optimal moisture in and around the wound surface for better revascularization and dermal regeneration. With several advantages, there are also a few disadvantages. A particular pressure of the water circulation is needed at the wound surface for rinsing of granulation tissue, which might impair the developing granulation tissue, restrict epidermal cell migration, and cause skin maceration [22]. In addition, bacterial infections can emerge if the moisture circulation is prolonged and proper drying of the wound is not done. Pulsed lavage therapy has currently become a replacement for hydrotherapy in terms of its use of an irrigating solution maintained at a particular pressure by a powered device [22]. The therapy improves the rate of granulation and better remodeling of wounded tissues. Ultraviolet C radiation (UV-C) ranges from 200–280 nm, and erythemal effectivity is accomplished at wavelengths of 250 nm where nucleic acid absorption happens, leading to accelerated DNA synthesis in fibroblasts, increased oxygenation and capillary blood flow for granulation tissue formation, and antibacterial and antiviral effects on wound surfaces. UV radiations can contribute to wound healing by upsurging epithelial cell turnover and hyperplasia to release prostaglandins and initiate cell proliferation for re-epithelialization. A dose-dependent application of these radiations may also cause shedding of peri-ulcer epidermal cells and sloughing of necrotic tissues and eschar [23]. Vacuum-assisted wound closure is applied to seal the wound area and place a negative pressure onto the wound surface that produces adhesive friction to the tissues and contracts wound depth for efficient closure [24]. This therapy can significantly and observably reduce water loss of the split-thickness graft area, curtail post-wounding duration, and restrict the relapse of infection during wound repair [24]. The accomplishment of vacuum-assisted closure therapy in treating chronic injuries has now led to its use in specialized clinical situations such as temporary abdominal closure, skin avulsion, poststernotomy mediastinitis, acute and subacute wounds, wound with bony prominence, osteomyelitis, as a graft reinforcement [24][25], and in reconstructive surgeries. Hyperbaric oxygen therapy confines a hundred percent oxygen at one atmospheric pressure to enhance oxygen inundation in the blood by forming oxyhemoglobin. Hyperoxic environments endorse wound repair through an increase in growth factors and formation of iNOS that regulates collagen formation, wound contraction, and endothelial progenitor cell proliferation [21][26]. This therapy has been utilized in chronic and poorly healing wounds, acute wounds, and diabetic foot ulcers. A systematic assessment of the healing capacity of hyperbaric oxygen therapy in diabetic foot ulcer patients was found to be clearly superior in comparison to other surgical procedures. Electrical simulation gathers both positive and negative charged cells, viz., neutrophils, phagocytes, epidermal cells, and fibroblasts, onto the wounded area so that each of the cells performs their specific cellular activities of wound healing. The endogenic electric field plays an imperative role in wound healing largely by triggering protein synthesis and cell migration. Clinical investigations have confirmed that electrical stimulation with steady direct currents is advantageous in wound acceleration. Human fibroblasts cells subjected to high-voltage pulsed current stimulation (HVPCS) did intensify the healing rate of soft tissue wounds as per reports [27]. Both protein and DNA synthesis rates became higher by applying specific blends of HVPCS voltages and pulse rates. Besides, both cell migration and a significant increase in the TGF-1β levels were prominent near the wound area in response to an endogenic electrical field that promoted early contraction and collagen synthesis (electrotaxis: the directional migration of cells toward the anodic or cathodic electrode of an applied electrical field) [27]. Researcher Yung Shin Sun experimentally aimed at optimizing the direct current stimulation therapy for enhancing the progression of wound repair. Via the finite element method (FEM), he standardized the distribution of endogenous electric fields produced around the wound area under the influence of different electrode configurations, including sizes and positions and the total power dissipation within different skin layers [28]. Extracorporeal shock wave therapy (ESWT) has been clinically relevant in being the cost-complaint effective intraoperative treatment for quite a few orthopedic and traumatic applications, together with challenging soft tissue wounds. These low-energy pulse waves were first clinically explored to treat urinary calcinosis, which later was utilized for treating bone and tendon injuries. Recently, ESWT has been studied to healing soft tissue wound injuries like burns, ulcers, etc., where these waves not only accelerate tissue repair, but also stimulate neovascularization in the underlying damaged tissues [29]. Additionally, these therapeutic shock waves also recruit newer mesenchymal stem cells, stimulate cell proliferation and differentiation, possess anti-inflammatory and antimicrobial effects, and block the toxic sensory pain receptors [29]. The underlying principle for ESWT employs short-term momentary acoustic pulsations with a simultaneous rise and fall in peak pressure time. The electro-hydraulic shock wave is generated within a metal inclusion, reflecting the shock waves towards the therapeutic target. Shock waves are electrically or mechanically produced energy waves that can traverse through a liquid or a gas medium. These waves either occur through electromagnetic, piezoelectric, or electrohydraulic wave pulses released from a high-voltage electrode water vaporization system [30]. During an injury, hypoxically challenged immunocompromised tissues are also subjected to ischemia. The application of ESWT onto the tissues increases tissue perfusion and reduces necrosis, as seen in experimental iatrogenic ischemia conditions [29]. Clinical research on experimental transgenic mice models displayed stimulation and upregulation of VEGF receptor 2, a mediator of angiogenesis, during ESWT treatments [31]. ESWT treatments also showed higher vessel densities in ischemic tissue formation experiments observed in immunohistopathological sections [32]. Another form of physical medication that uses low-level lasers or light-emitting diodes onto the body surface, known as the low-level laser therapy (LLLT), which has been effectively used as a therapeutic system to repair deferred wounds. This photothermal method comprises a monochromatic and coherent light source that produces a healing effect when the emitted photons are absorbed onto the wavelength-sensitive injured tissues/cells and trigger a set of complex biochemical arrays, ultimately resulting in accelerated healing of chronic wounds [33]. Lasers also employ their applications in burns, amputation wounds, skin grafting, infected wounds, etc. [34]. Low-intensity irradiations work between 500 and 1100 nm with laser output powers between 10 and 90 mW at the therapeutic site, capable of mediating cell proliferation and enhancing cellular activity. Visible red light, having the longest wavelength, is frequently used in LLLT, and has been seen to trigger faster healing of tissues by irradiating the mitochondrial complex and plasmalemma of the damaged cells, where the absorbed photons activate a set of enzymatic reactions and cytochromes, and energy conversions happen to yield adenosine triphosphates that, in turn, regulate cell function, relieve inflammation and pain, and accelerate healing [34]. Reports on the growth inhibition of Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus upon irradiation with 1–20 J/cm2 at wavelengths of 630 nm were seen in preventing wound infections [33]. LLLT may also show substantial positive results in stimulation and co-adjuvanted therapies. A study conducted by Pessoa et al. to inspect the co-effect of LLLT with steroid cortisone did show a co-adjuvanted effect of LLLT on cortisone to not only speed up the tissue healing process, but also to stabilize the harmful effects of steroids on tissues [35]. Neat corticosteroid, neat 5-fluorouracil, a pulsed dye laser (585-nm), and the amalgamation of all the three showed a significant enhancement in keloidal and hypertrophic scars post-treatment. Pulsed dye laser enhanced the scar surface and improved long-term adverse hypopigmentation, telangiectasia, and skin atrophy, unlike the corticosteroid [36].
2. Engineered Metal Composites Implemented for Healing
The nanoceutical adjuvants are biological entities that use nanotechnology to enhance their properties and garner their usage in diverse nano-based regenerative medicine applications. Nanoceutical materials possess lucrative physicochemical characteristics, which makes them of particular interest in various biomedical applications. Though scientific evidence regarding safety or efficacy is still being explored, the number of commercially viable nanoceuticals has magnified to a large extent. The growth trend of nanoceutical products is expected to continue and facilitate personalized medicine, targeted therapies with reduced side effects, and artificial intelligence-aided patient monitoring. However, mega challenges persist, particularly regarding biodistribution, metabolism in organs, and excretion from the body. Nanoceutical materials comprise designed metal-based nanoparticles and biomaterials that offer an unmatched approach to accelerate wound repair and the tissue-remodeling process. Their dimensions and shape govern their specificity, biological efficacy, cellular response, penetrability, and targeted delivery to the site of injury. These are comparatively nontoxic and exhibit high antibacterial properties [37]. In addition, other nanoceutical counterparts, such as the nanospheres, nano-capsules, nano-emulsions, nanocarriers, nano-scaffolds/composites, and nano-colloids, could serve as materials for wound tissue regeneration. The main focus here is metal-based nanoparticles as nanoceutical adjuvants for healing, followed by a brief overview of the other types.
Nanoparticles, both metallic and non-metallic, principally aid in wound repair and management by either possessing inbuilt inherent features that assist wound contraction or as delivery vehicles/carriers for assisted therapy. The most extensively studied ones are silver, gold, and zinc nanoparticles, owing to their unique dimensional, functional, chemical, and biochemical properties [37]. Silver nanoparticles (AgNPs), the potential candidate of choice for wound repair, have a high surface:volume ratio, show excellent activities at low concentrations, and are superior to the traditional silver compounds that were formerly used. Neat AgNPs can regulate the release of anti-inflammatory cytokines that facilitate rapid non-hypertrophic scar-devoid wound contraction [37][38]. AgNPs can initiate proliferation and differentiation of keratinocytes to augment epidermal closure and re-epithelialization and antibacterial efficacy. Myofibroblast differentiation from normal fibroblasts to promote speedy tissue renewal is also facilitated by AgNPs [37]. However, reports on their toxicity at increased concentrations by Szmyd et al. has shown that keratinocyte feasibility, absorption, migration, and differentiation are affected via specific cell death initiating the stimulus of caspase 3 and 7, ultimately leading to DNA mutilation [39]. Therefore, it is recommended to use lower safe doses, together with antimicrobic preparations (in the form of heat, radiation, and natural and synthetic chemicals, viz., phytochemicals, antiseptics, antibiotics, polymers, etc.), to attain improved effectiveness. AgNPs in conglomeration with the polyketide antibiotic can significantly reduce the bacterial load in epidermal and deep dermal layers in a mice model and quicken healing [38][39]. Hence, the combination of nanoparticles with conventional antibacterial mediators or dressings can more competently be used to repair infected wounds. Micro-cellulose reinforced with AgNPs behaves as an antimicrobial coating for open wounds and has shown high antibacterial performance against Gram-negative pathogens [40]. Experimental evidence by Chakrabarti et al. also depicted that coated polyester-nylon dressings with AgNPs can prevent biofilm formation and bacterial colonization, while upholding a low toxicity profile [40]. AgNPs form sulfur bonds with the bacterial plasmalemma proteins or bind to enzymatic thiol moieties, resulting in respiratory chain reactions and cell death [39]. Furthermore, these nanoparticles can hinder DNA synthesis and curb bacterial multiplication in the wounds. Experimental observations by Lu et al. showed that the incorporation of silica into AgNPs could give rise to nontoxic mesoporous disulfide structures, which can very efficiently adhere to open wounds and have outstanding bacteriostatic activity [37]. Ag-infused veneers warrant quicker wound healing and evade microbial colonization on the wound site, as observed in an in vivo canine model [37]. Commercially available silver-impregnated dressings, Acticoat ™ nano-sized AgNPs (<15 nm size) are currently being explored for their reparative, anti-infective, and pain-lessening facets for early burn wounds and may be complaint in evading burn wound infections upon their amalgamation with silver sulphadiazine and chlorhexidine digluconate formulation [41].
Gold nanoparticles (AuNPs) show potency in tissue rejuvenation, targeted drug delivery, and wound repair due to their extraordinary biocompatibility. AuNPs nanomaterials, because of their stabilizing properties, can be used as reinforcements with many other nanomaterials. Since neat gold particles do not exhibit a substantial activity, they need to be incorporated or combined with some matrix or therapeutic agent, a carrier, biomolecules, etc., for efficient antimicrobial activity. The cross-linking of AuNPs with collagen, chitosan, gelatin, and alginate, and their incorporation with various polysaccharides, growth factors, peptides, and cell adhesion proteins enables their attachment onto the gold nanoparticle surface without any alteration in the structural conformation of the biomolecule. These conglomerated moiety-modified AuNPs display excellent biocompatibility and biodegradability and are suited for healing. Similar to collagen, gelatin and chitosan can also easily be incorporated with AuNPs, showing safe and positive effects in enhancing wound healing [37][38]. Additionally, by modifying the surface plasmon resonance of AuNP, these exhibit thermo-responsive behavior, which is supported by in vitro and in vivo experimental data [37]. The mechanism of action of AuNPs follows either targeting the cell wall or binding to DNA to stall the double-helical structure from unwinding during replication or transcription, therefore contributing to bactericidal and bacteriostatic activities. They can thus show multidrug resistance to Staphylococcus aureus and Pseudomonas aeruginosa. AuNPs are also potent antioxidants [42]. Low concentrations of AuNPs are associated with keratinocyte growth and differentiation [37]. Observations made by Marza et al., on basic fibroblast growth factor AuNP-impregnated petroleum jelly mixtures showed enhanced angiogenesis and fibroblast proliferation, which aided speedy wound recovery [43]. The effect of colloidal AuNP coupled with quercetin (AuQurNPs) on the fibroblast cell migration-assisted wound healing mechanism was depicted by Madhyastha et al. AuQurNPs displayed enhanced cell proliferation and migration of keratinocytes, which was directed through the TGF-β1-dependent SMAD signaling pathway. This initial study on nanoceutical-engineered gold particles brings forth molecular and cellular evidence-based data to elevate the promising healing applications of AuQurNPs in upcoming nanomedicine for skin etiology [44] (Figure 1).
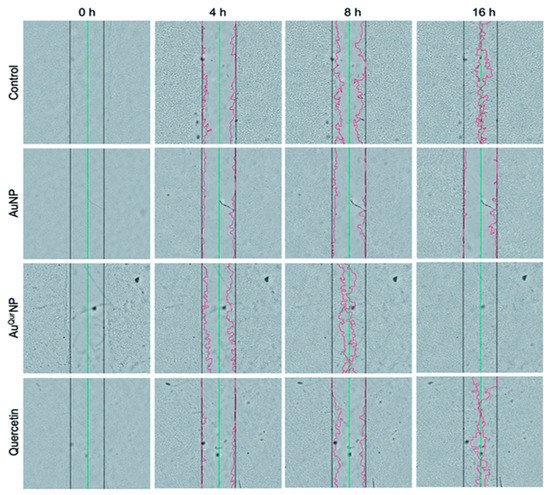
Figure 1. In vitro wound assay of human keratinocyte cells treated with AuNP (5 μg l−1), AuQurNP (5 μg l−1), or pure quercetin (15 ng ml−1) for different time periods (0, 4, 8, 16 h). Non-treated cells were used as control. Black, green, and red lines depict the start, end-point of cell migration, and migratory cell edge, respectively (magnification:10X). (reprinted with permission from reference [44]. Reproduced with permission of The Royal Society of Chemistry).
Zinc-oxide nanoparticles (ZnONPs) exhibit potent antibacterial activity, and combined with hydrogel-based wound dressings [45], can activate keratinocyte migration and improve re-epithelialization [46] (Figure 2). A recent study on the assessment of ZnONP-based chitosan hydrogel formulations presented high absorbency of wound exudates and aided hemostatic blood clotting and antibacterial effectivity simultaneously [7]. ZnONPs and a collagen-based bioresorbable matrix with orange essential oil have been seen to substantially heal burn wounds, while also decreasing sepsis chances. This wound dressing was seen to augment angiogenesis, form new tissue, and exhibit biocompatibility and no cytotoxicity when evaluated in vitro and in vivo [47]. Yet, their inherent toxicity makes them less used in wound healing therapies [7]. ZnONP toxicity is dose-dependent, with higher doses it acts as a mitochondrial-dysfunctioning agent to release reactive oxygen species and block gene expression of superoxide dismutase and glutathione peroxidase in human keratinocytes, ultimately giving rise to oxidative stress and cell death. Creating core-shell nanocomposites by combining two metals, such as biogenic AuNPs with a thin coat of ZnO to form AuZnO core-shell nanocomposites, was assessed to evaluate the antibacterial and anti-biofilm efficacy against Staphylococcus aureus and methicillin-resistant Staphylococcus haemolyticus [48]. ZnONPs also have good tissue adhesive properties, as exhibited in mice skin models [37].
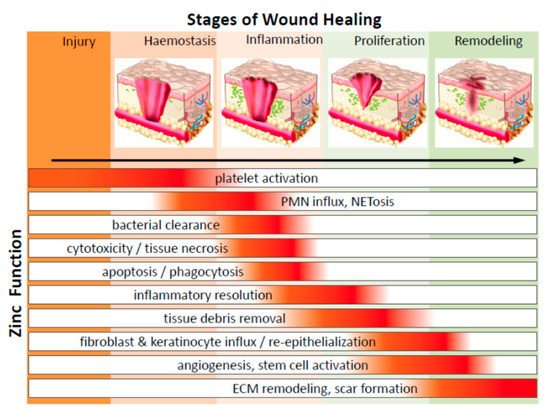
Figure 2. Diagrammatic representation of the significant function of zinc on cells in various phases of wound healing (PMN—polymorphonuclear leukocytes; NETosis—a novel form of programmed neutrophil death that resulted in the formation of neutrophil extracellular traps (NETs)) (adapted from Reference [47] respectively, open access).
Nanoparticle-based composites: Renewable sources for nanoparticle synthesis and the development of its composites are gaining increased importance as they are cost-effective, consume less energy, and do not require additional sources for disposing of toxic by-products, unlike in nanoparticle synthesis via a chemical process. Several phytochemicals, viz., alkaloids, phenols, amino acids, proteins, etc., have been used to stabilize several nanoparticles like Ag ions in AgNPs. Ocimum sanctum merged AgNPs embedded into a Carbopol gel base and attained ≈96% of wound contraction by the 14th post-wounding day, as revealed by Sood and coworkers [49]. Additionally, they possessed activity against Staphylococcus aureus and Pseudomonas aeruginosa. Gelatin has in situ reductive properties to stabilize AgNPs, and the development of gelatin-chitosan-Ag porous composites with sizes 100–250µm that are biocompatible, biodegradable, and non-immunogenic, which upon cross-linking with tannic acid does exhibit therapeutic and antibacterial characteristics with low cytotoxicity [50]. Shao et al. [51] used Barleria gibsoni leaf extracts to synthesize ZnONPs gel formulation that showed substantial effect against Gram-positive and Gram-negative infected burn wounds. Polymers such as chitosan can also serve as nanomaterial dressings or drug carriers due to their biocompatible polymeric networks to hold optimum moisture for a balanced wound environment. Chitosan, being cationic, attract most metals, proteins, and dyes to form complexes [52], and its degradation products can activate ECM synthesis. Chitosan-assisted wound healing therapies include hydrogels, membranes, films, sponges, and scaffolds [52]. Chitosan nanoparticles have immunomodulatory and nontoxic effects on human dermal cells, as revealed by Chen et al. in his assembled acellular porcine dermal matrix using a naturally-derived chitosan oligosaccharide [53]. Additionally, the presence of the functional group aldehyde in AgNPs, formed during in situ reaction, confers the chitosan-AgNPs scaffolds with broad-spectrum antimicrobial properties against associated Escherichia coli and Staphylococcus aureus [54]. A combination of a chitosan-poly (vinyl alcohol) (PVA) complex improved the antioxidant and antimicrobial efficacy compared to the polymer alone. It also conferred strong Gram-negative activity against Klebsiella species and further enhanced in vivo wound repair by forming granulation tissue and re-epithelialization while demonstrating no cytotoxicity [54]. An infrared-irradiation-triggered thermo-sensitive hydrogel-based drug delivery system loaded with ciprofloxacin was developed by Gao et al., triggered by near-infrared light stimulation. The mixture of polydopamine nanoparticles/glycol chitosan, being photothermally active, generated hyperthermia leading to bacterial cell leaching. Besides, polydopamine nanoparticles in combination with the drug ciprofloxacin exhibit a controlled released when stimulated with near-infrared light and showed minimal leakage under physiological conditions [54]. Calreticulin (calcium-binding protein)-based AuNPs and chitosan/AuNP nanocomposites have been used for diabetic lesions. Calreticulins regulate the proper folding of proteins, and the nanocomposite endorses fibroblast-keratinocyte-endothelial cell growth, migration, division, and collagen formation without hindering cell proliferation [55]. The biopolymer cellulose triggers repair via multiple local growth factors such as the epidermal growth factor and basic fibroblast growth factor [56]. Nanocellulose dressings, due to their anti-infective properties and amplified tensile properties, have been explored as scaffolds [7]. Bacterial cellulose mimics the skin structure with a high surface area per unit, increased biocompatibility, hydrophilicity, and no cytotoxicity. 3D porous networks of nanocellulose have high water retention capacity, ensuring a moist environment appropriate for healing [7]. The wound healing potential of cellulose-ZnONPs composites was displayed by Khalid et al. [56]. Bacterial nanocellulose derived from Gram-negative Gluconacetobacter xylinus, and combined with silver nanoparticles, showed enhanced healing and reduced colonization of wound-associated Staphylococcus aureus in vitro [7].
Nanoparticle-embedded nano-scaffold systems: Nanoparticles in nano-scaffold systems for wound healing applications have escalated in the last few years. Nano-scaffolds, or better elaborated as nanofibrous scaffolds, are nano-systems premeditated to resemble the components of the cellular microenvironment or to reorientate cell behavior. The predicted tendency is to use biodegradable biomaterials, which help regenerate and repair damaged tissue [57]. With advanced tissue engineering tools such as nano-architectonics approaches, in which materials are designed taking into account methods including organic synthesis, self-assembly/self-organization, molecular manipulation, and structural regulation upon stimuli, scaffolds are being tailor-made to have good biodegradability, mechanical properties, and ease in processing some polymers to better fit into different tissue engineering arenas. At present, only a few techniques can successfully produce nano-scaffolds, and their consequent nanomaterials, within the nanoscale frame [58]. Electrospinning is among the few methods that results in uniform and stable morphological nanofibrous scaffolds [59]. The amalgamation of bioactives by direct dissolution into functional polymer solutions helps progress healing at different phases. Further, nanopolymers like the dendrimers also show anti-inflammatory and antibacterial characteristics. Studies on a porcine model of superficial partial-thickness wounds displayed enhance healing potency of an electrospun polymer nanofiber dressing with the least risk of infection. Chitosan-poly-vinyl alcohol nanofibrous scaffolds upon application to rat diabetic wound models had improved healing rates compared to controls [60]. An in vivo study in Wistar rats of a silver nanoparticle-spun nanofiber membrane exhibited numerous favorable effects of reduced cytotoxicity, broad-spectrum antibacterial action, abridged inflammation, and higher healing rates [61]. Recombinant human epidermal growth factor, another nanocarrier, has been revealed to stimulate healing of full-thickness diabetic wounds. Nevertheless, their restricted use is due to the highly proteolytic environment they possess and the downregulation of associated growth factor receptors and signaling molecules in the case of chronic wounds [62]. However, the results vary between experiments. Zhang et al. [63] defined a hydrogel with Ca2+ cross-linker as capable of releasing preloaded bFGF. Observations that both calcium and bFGF led to the growth and division of fibroblasts in the early re-epithelialization phases, persuading wound shrinkage on both in vitro and in vivo models. Nanofibrous mesh networks developed by electrospinning have been used for gene encapsulation in wound dressings. Gene-activated matrix therapy can simultaneously alter the expression of a target gene involved in regeneration and bridge the gap between tissue engineering and gene therapy. Wang et al. [64] optimized a gene delivery system based on the antimicrobial peptide LL-37 embedded on ultra-small AuNPs, which increased the complete antibacterial action in the topical treatment of diabetic lesions. Furthermore, a LL37-AuNPs composite boosted cellular and nucleus diffusion, thus accomplishing high gene delivery efficacy. This system possessed biocompatibility, endorsed angiogenesis through the expression of VEGF expression, and improved re-epithelialization and granulation tissue formation [65]. Nanoceria has scavenging activity due to the coexistence of two oxidation states (3+ and 4+) in the valence cerium atom. Hence, these nanoparticles may diminish oxidative stress and reinstate the balance between oxidants and antioxidant enzymes in diabetic lesions. A 100µg dose of cerium oxide nanoparticles-miR-146a combination enhanced diabetic wound healing without altering the wound tensile [65]. Stem cell therapy, another feather in the cap of tissue engineering and regenerative medicine, represents another possible beneficiary of the nano scaffold technology due to their substantial stem cell migration and differentiation. These multifaceted nanomaterials with numerous enhancing properties represent advantages compared to standard treatment procedures adopted in clinical practice.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094748
References
- Singh, M.; Nuutila, K.; Kruse, C.; Robson, M.C.; Caterson, E.; Eriksson, E. Challenging the conventional therapy: Emerging skin graft techniques for wound healing. Plast. Reconstr. Surg. 2015, 136, 524e–530e.
- Kaufman, C.L.; Bhutiani, N.; Ramirez, A.; Tien, H.Y.; Palazzo, M.D.; Galvis, E.; Farner, S.; Ozyurekoglu, T.; Jones, C.M. Current status of vascularized composite allotransplantation. Am. Surg. 2019, 85, 631–637.
- Benichou, G.; Yamada, Y.; Yun, S.H.; Lin, C.; Fray, M.; Tocco, G. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy 2011, 3, 757–770.
- Wyburn, K.R.; Jose, M.D.; Wu, H.; Atkins, R.C.; Chadban, S.J. The role of macrophages in allograft rejection. Transplantation 2005, 80, 1641–1647.
- Ouimet, M.; Ediriweera, H.N.; Gundra, U.M.; Sheedy, F.J.; Ramkhelawon, B.; Hutchison, S.B.; Rinehold, K.; van Solingen, C.; Fullerton, M.D.; Cecchini, K.; et al. MicroRNA-33–dependent regulation of macrophage metabolism directs immune cell polarization in atherosclerosis. J. Clin. Investig. 2015, 125, 4334–4348.
- Ekser, B.; Ezzelarab, M.; Hara, H.; van der Windt, D.J.; Wijkstrom, M.; Bottino, R.; Trucco, M.; Cooper, D.K. Clinical xenotransplantation: The next medical revolution? Lancet 2012, 379, 672–683.
- Ovington, L.G. Advances in wound dressings. Clin. Dermatol. 2007, 25, 33–38.
- Fonder, M.A.; Mamelak, A.J.; Lazarus, G.S.; Chanmugam, A. Occlusive wound dressings in emergency medicine and acute care. Emerg. Med. Clin. N. Am. 2007, 25, 235–242.
- Ghalei, S.; Nourmohammadi, J.; Solouk, A.; Mirzadeh, H. Enhanced cellular response elicited by addition of amniotic fluid to alginate hydrogel-electrospun silk fibroin fibers for potential wound dressing application. Colloids Surf. B 2018, 172, 82–89.
- Aderibigbe, B.A.; Buyana, B. Alginate in wound dressings. Pharmaceutics 2018, 10, 42.
- Wu, S.; Deng, L.; Hsia, H.; Xu, K.; He, Y.; Huang, Q.; Peng, Y.; Zhou, Z.; Peng, C. Evaluation of gelatin-hyaluronic acid composite hydrogels for accelerating wound healing. J. Biomater. Appl. 2017, 31, 1380–1390.
- Derwin, R.; Moore, Z.E.; Webster, J. Hydrocolloid dressings for donor sites of split thickness skin grafts. Cochrane Database Syst. Rev. 2018.
- Walker, R.M.; Gillespie, B.M.; Thalib, L.; Higgins, N.S.; Whitty, J.A. Foam dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 10.
- Singer, A.J.; Clark, R.A.F. Mechanisms of disease: Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746.
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound healing and the use of medicinal plants. Evid. Based Complement. Altern. Med. 2019.
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10, 9–14.
- Thomas, G.W.; Rael, L.T.; Bar-Or, R.; Shimonkevitz, R.; Mains, C.W.; Slone, D.S.; Craun, M.L.; Baror, D. Mechanisms of delayed wound healing by commonly used antiseptics. J. Trauma Acute Care Surg. 2009, 66, 82–91.
- Noda, Y.; Fujii, K.; Fujii, S. Critical evaluation of cadexomer-iodine ointment and povidone-iodine sugar ointment. Int. J. Pharm. 2009, 372, 85–90.
- Atiyeh, B.S.; Dibo, S.A.; Hayek, S.N. Wound cleansing, topical antiseptics and wound healing. Int. Wound J. 2009, 6, 420–430.
- Alves, P.J.; Barreto, R.T.; Barrois, B.M.; Gryson, L.G.; Meaume, S.; Monstrey, S.J. Update on the role of antiseptics in the management of chronic wounds with critical colonisation and/or biofilm. Int. Wound J. 2020.
- Hess, C.L.; Howard, M.A.; Attinger, C.E. A review of mechanical adjuncts in wound healing: Hydrotherapy, ultrasound, negative pressure therapy, hyperbaric oxygen, and electrostimulation. Ann. Plast. Surg. 2003, 51, 210–218.
- Atkin, L.; Ousey, K. Wound bed preparation: A novel approach using hydrotherapy. Br. J. Community Nurs. 2016, 21, S23–S28.
- Gupta, A.; Avci, P.; Dai, T.; Huang, Y.Y.; Hamblin, M.R. Ultraviolet radiation in wound care: Sterilization and stimulation. Adv. Wound Care 2013, 2, 422–437.
- Herscovici, D.; Sanders, R.W.; Scaduto, J.M.; Infante, A.; DiPasquale, T. Vacuum-assisted wound closure (VAC therapy) for the management of patients with high-energy soft tissue injuries. J. Orthop. Trauma 2003, 17, 683–688.
- Sinha, K.; Chauhan, V.D.; Maheshwari, R.; Chauhan, N.; Rajan, M.; Agrawal, A. Vacuum assisted closure therapy versus standard wound therapy for open musculoskeletal injuries. Adv. Orthop. 2013.
- Copeland, H.; Newcombe, J.; Yamin, F.; Bhajri, K.; Mille, V.A.; Hasaniya, N.; Bailey, L.; Razzouk, A.J. Role of negative pressure wound care and hyperbaric oxygen therapy for sternal wound infections after pediatric cardiac surgery. World J. Pediatr. Congenit. Heart Surg. 2018, 9, 440–445.
- Thakral, G.; LaFontaine, J.; Najafi, B.; Talal, T.K.; Kim, P.; Lavery, L.A. Electrical stimulation to accelerate wound healing. Diabet. Foot Ankle 2013, 4, 22081.
- Sun, Y.S. Electrical stimulation for wound-healing: Simulation on the effect of electrode configurations. Biomed Res. Int. 2017.
- Schaden, W.; Thiele, R.; Kölpl, C.; Pusch, M.; Nissan, A.; Attinger, C.E.; Maniscalco-Theberge, M.E.; Peoples, G.E.; Elster, E.A.; Stojadinovic, A. Shock wave therapy for acute and chronic soft tissue wounds: A feasibility study. J. Surg. Res. 2007, 143, 1–12.
- Mouzopoulos, G.; Stamatakos, M.; Mouzopoulos, D.; Tzurbakis, M. Extracorporeal shock wave treatment for shoulder calcific tendonitis: A systematic review. Skelet Radiol. 2007, 36, 803–811.
- Mittermayr, R.; Hartinger, J.; Antonic, V.; Meinl, A.; Pfeifer, S.; Stojadinovic, A.; Schaden, W.; Redl, H. Extracorporeal shock wave therapy (ESWT) minimizes ischemic tissue necrosis irrespective of application time and promotes tissue revascularization by stimulating angiogenesis. Ann. Surg. 2011, 253, 1024–1032.
- Yan, X.; Zeng, B.; Chai, Y.; Luo, C.; Li, X. Improvement of blood flow, expression of nitric oxide, and vascular endothelial growth factor by low-energy shockwave therapy in random-pattern skin flap model. Ann. Plast. Surg. 2008, 61, 646–653.
- Hawkins, D.; Houreld, N.; Abrahamse, H. Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. Ann. N. Y. Acad. Sci. 2005, 1056, 486–493.
- Schindl, A.; Schindl, M.; Pernerstorfer-Schön, H.; Schindl, L. Low-intensity laser therapy: A review. J. Investig. Med. 2000, 48, 312–326.
- Pessoa, E.S.; Melhado, R.M.; Theodoro, L.H.; Garcia, V.G. A histologic assessment of the influence of low-intensity laser therapy on wound healing in steroid-treated animals. Photomed. Laser Surg. 2004, 22, 199–204.
- Manuskiatti, W.; Fitzpatrick, R.E. Treatment response of keloidal and hypertrophic sternotomy scars: Comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch. Dermatol. 2002, 138, 1149–1155.
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Materials 2019, 12, 2176.
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612.
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.R.; Vindigni, V.; Azzena, B.; Barbante, C.; et al. Active silver nanoparticles for wound healing. Int. J. Mol. Sci. 2013, 14, 4817–4840.
- Chakrabarti, S.; Chattopadhyay, P.; Islam, J.; Ray, S.; Raju, P.S.; Mazumder, B. Aspects of nanomaterials in wound healing. Curr. Drug Deliv. 2019, 16, 26–41.
- Fong, J.; Wood, F.; Fowler, B. A silver coated dressing reduces the incidence of early burn wound cellulitis and associated costs of inpatient treatment: Comparative patient care audits. Burns 2005, 31, 562–567.
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical intermediate-modified gold nanoparticles: Against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 2017, 11, 5737–5745.
- Marza, S.; Magyari, K. Skin wound regeneration with bioactive glass-gold nanoparticles ointment. Biomed. Mater. 2019, 14, 025011.
- Madhyastha, H.; Halder, S.; Madhyastha, R.; Mohanapriya, A.; Sudhakaran, R.; Sajitha, L.S.; Banerjee, K.; Bethasiwi, P.; Daima, H.; Navya, P.N.; et al. Surface refined Au Quercetin nanoconjugate stimulates dermal cell migration: Possible implication in wound healing. RSC Adv. 2020, 10, 37683–37694.
- Woo, K.Y.; Sibbald, R.G. The improvement of wound-associated pain and healing trajectory with a comprehensive foot and leg ulcer care model. J. Wound Ostomy Cont. Nurs. 2009, 36, 184–191.
- Balaure, P.C.; Holban, A.M.; Grumezescu, A.M.; Mogo¸sanu, G.D.; Bal¸seanu, T.A.; Stan, M.S.; Dinischiotu, A.; Volceanov, A.; Mogoanta, L. In vitro and in vivo studies of novel fabricated bioactive dressings based on collagen and zinc oxide 3D scaffolds. Int. J. Pharm. 2018, 557, 199–207.
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.; Steinberg, S.M.; Ma, J. Zinc in wound healing modulation. Nutrients 2018, 10, 16.
- Khan, M.I.; Behera, S.K.; Paul, P.; Das, B.; Suar, M.; Jayabalan, R.; Fawcett, D.; Poinern, G.E.J.; Tripathy, S.K.; Mishra, A. Biogenic core-shell nanocomposites kill Staphylococcus aureus without provoking nuclear damage and cytotoxicity in mouse fibroblasts cells under hyperglycemic condition with enhanced wound healing proficiency. Med. Microbiol. Immunol. 2018.
- Sood, R.; Chopra, D.S. Optimization of reaction conditions to fabricate Ocimum sanctum synthesized silver nanoparticles and its application to nano-gel systems for burn wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 575–589.
- Ye, H.; Cheng, J.; Yu, K. In situ reduction of silver nanoparticles by gelatin to obtain porous silver nanoparticle/chitosan composites with enhanced antimicrobial and wound-healing activity. Int. J. Biol. Macromol. 2019, 121, 633–642.
- Shao, F.; Yang, A.; Yu, D.M.; Wang, J.; Gong, X.; Tian, H.X. Bio-synthesis of Barleria gibsoni leaf extract mediated zinc oxide nanoparticles and their formulation gel for wound therapy in nursing care of infants and children. J. Photochem. Photobiol. B 2018, 189, 267–273.
- Biranje, S.S.; Madiwale, P.V.; Patankar, K.C.; Chhabra, R.; Dandekar-Jain, P.; Adivarekar, R.V. Hemostasis and anti-necrotic activity of wound-healing dressing containing chitosan nanoparticles. Int. J. Biol. Macromol. 2019, 121, 936–946.
- Chen, Y.; Dan, N.; Dan, W.; Liu, X.; Cong, L. A novel antibacterial acellular porcine dermal matrix cross-linked with oxidized chitosan oligosaccharide and modified by in situ synthesis of silver nanoparticles for wound healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 1020–1036.
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Wu, F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2019, 188, 83–95.
- Hernandez Martinez, S.P.; Rivera González, T.; Franco Molina, M.; Bollain y Goytia, J.; Martínez Sanmiguel, J.; Zárate Triviño, D.; Rodríguez Padilla, C. A Novel Gold Calreticulin Nanocomposite Based on Chitosan for Wound Healing in a Diabetic Mice Model. Nanomaterials 2019, 9, 75.
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221.
- Alberti, T.B.; Coelho, D.S.; de Prá, M.; Maraschin, M.; Veleirinho, B. Electrospun PVA nanoscaffolds associated with propolis nanoparticles with wound healing activity. J. Mater. Sci. 2020, 55, 9712–9727.
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433.
- An, J.; Chua, C.K.; Yu, T.; Li, H.; Tan, L.P. Advanced nanobiomaterial strategies for the development of organized tissue engineering constructs. Nanomedicine 2013, 8, 591–602.
- Dong, R.H.; Jia, Y.-X.; Qin, C.-C.; Zhan, L.; Yan, X.; Cui, L.; Zhou, Y.; Jiang, X.; Long, Y.-Z. In situ deposition of a personalized nanofibrous dressing via a handy electrospinning device for skin wound care. Nanoscale 2016, 8, 3482–3488.
- Gholipour-Kanani, A.; Bahrami, S.H.; Rabbani, S. Effect of novel blend of nanofibrous scaffolds on diabetic wounds healing. IET Nanobiotechnol. 2016, 10, 1–7.
- Chu, Y.; Yu, D.; Wang, P.; Xu, J.; Li, D.; Ding, M. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen. 2010, 18, 499–505.
- Zhang, X.; Kang, X.; Jin, L.; Bai, J.; Liu, W.; Wang, Z.; Ji, L. Stimulation of wound healing using bioinspired hydrogels with basic fibroblast growth factor (bFGF). Int. J. Nanomed. 2018, 13, 3897–3906.
- Wang, S.; Yan, C.; Zhang, X.; Shi, D.; Chi, L.; Luo, G.; Deng, J. Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater. Sci. 2018, 6, 2757–2772.
- Zgheib, C.; Hilton, S.A.; Dewberry, L.C.; Hodges, M.M.; Ghatak, S.; Xu, J.; Singh, S.; Roy, S.; Sen, C.K.; Seal, S.; et al. Use of cerium oxide nanoparticles conjugated with MicroRNA-146a to correct the diabetic wound healing impairment. Lasers Med. Sci. 2019, 228, 107–115.
