Nanoscience and nanotechnology have revolutionized key areas of environmental sciences, including biological and physical sciences. Nanoscience is useful in interconnecting these sciences to find new hybrid avenues targeted at improving daily life. Pharmaceuticals, regenerative medicine, and stem cell research are among the prominent segments of biological sciences that will be improved by nanostructure innovations. Nanoparticles, nanowires, hybrid nanostructures, and nanoscaffolds, that have been useful in mice for ocular tissue engineering and regeneration.
- nanoparticles
- nanodisks
- scaffolds
- nano-biomaterials and retina
- nanoscaffolds and retinal regeneration
- nanoparticles and retinal regeneration
1. Introduction
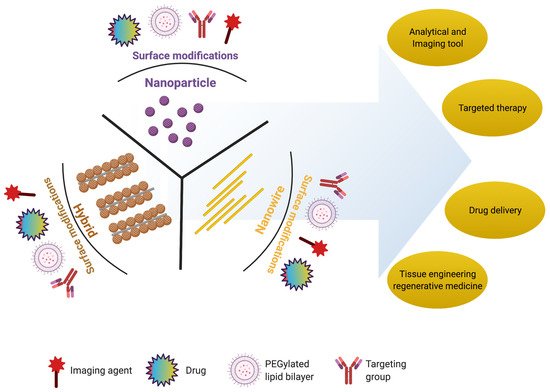
2. Nanomaterials for Retinal Regeneration
In the present section, we will discuss the importance of nanoparticles, nanowires, and hybrid nanostructures in retinal regeneration, summarized in Table 1.
| Nanostructure | Nanomaterial | Size Range (nm) | Target Tissue/Cells | Ref. |
|---|---|---|---|---|
| Nanoparticles | Gold (Au) (diameter) |
3–5 | Choroidal and retinal endothelial cells | [31] |
| 10–12 | Retina of rabbit | [32] | ||
| 10–20 | Photoreceptor precursor transplantation | [33] | ||
| 80 | Retinal cells | [34] | ||
| 20–80 | Nucleus and mitochondria of retinal cells | [35] | ||
| 5–20 | Blood–retinal barrier | [33][36][37] | ||
| Gold (Au) nanodisk | Thickness: 20 Diameter: 160 |
Retina | [36] | |
| Silver (Ag) (diameter) |
20–80 | Bovine retinal endothelial cells | [38] | |
| 40–50 | Porcine retinal endothelial cells | [39] | ||
| Superparamagnetic iron oxide nanoparticles | Diameter: 5–20 |
Retina | [40] | |
| Magnetite | 10 | Retina and cells | [41][42] | |
| NWs | Poly (ε-caprolactone) (PCL) membranes | Length: 2500 |
Implantation into subretinal space | [43] |
| Gallium phosphide (GaP) | Length: 500–4000 |
Retinal cells | [44] | |
| n-type silicon | Length: 4400 |
Retinal cells | [45] | |
| Gold (Au) nanorods | Thickness: 10–35 |
Retinal cells and photoreceptors | [46] | |
| Hybrid nanostructure | Gold NPs coated over titania (TiO2) NWs |
Au NP diameter: 5–15 TiO2 NW length: 2000 |
Artificial photoreceptors | [43][47][48][49][50] |
| Gallium phosphide (GaP) rod and cone | Length: 20–2500 |
Ganglion cells, and bipolar cells | [44] | |
| Gold NPs coated over silicon NWs |
Au NP diameter: 5–10 NW length: 500–2500 |
Artificial photoreceptors | [51][52] | |
| Thin film functionalized with the NPs | Diameter: 5–50 |
Photoreceptors | [53][54] | |
| p–n junction silicon NWs | NW length: 10–120 |
Membranes of live bipolar cells | [55] | |
| Au-coated carbon nanotube (Au-CNT) | Au NP diameter: 5–20 CNT length: 500–2500 |
Subretinal space of mice | [56] | |
| Iridium oxide (IrOx) combined with reduced graphene oxide | IrOx diameter: 2–25 CNT length: 2–2500 |
Subretinal implant into live mice | [57] | |
| Iridium oxide (IrOx) coated with CNT | IrOx diameter: 5–25 CNT length: 500–2500 |
Retinal cells/tissues | [41][58][59][60][61] | |
| Core–shell-structured β-NaYF4:20%Yb, 2%Er@β-NaYF4 nanoparticles | Diameter: 30–40 |
Subretinal space of mice | [4] | |
| Nanoscaffolds | Natural polymer: gelatin, fibrin, chitosan, laminin, and hyaluronic acid |
Diameter/porosity: 100–200 |
Extracellular matrix and cell attachment | [62][63][64][65][66][67] |
| Synthetic polymer: poly (lactic-co-glycolic acid) (PLGA), poly (ε-caprolactone) (PCL), poly (L-lactic acid) (PLA), polyimide, and poly (l-lactide-co-ε-caprolactone) |
Diameter/porosity: 50–500 |
RPE, biological activity, extracellular matrix, and cell attachment | [68][69][70][71] | |
| Biohybrid: nanofibers of Bruch’s membrane |
Diameter/porosity: 100–200 |
RPE and biological activity | [72] |
2.1. Nanoparticles
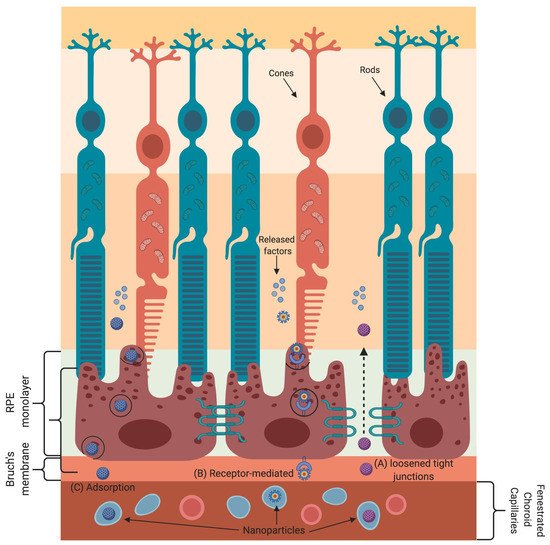
Nanoparticle-based gene and drug delivery to retinal cells has been harnessed to treat various eye diseases [33][73][74][75][76][77][78][79][80]. The various transport mechanisms that nanoparticles employ to cross the blood–retinal barrier are shown in Figure 2. Nanoparticles absorb or scatter light at specific frequencies/wavelengths as a function of their physical and chemical characteristics. These properties of nanoparticles are well suited for bioimaging and to treat cancer by using near-infrared-triggered photothermic therapy (PTT) [81]. Due to the low absorption coefficients of hemoglobin and water, the penetration of near-infrared (650–900 nm) rays in tissues is very high, allowing the use of near-infrared rays for nanoparticle stimulation without damaging the tissue [82]. Gold-nanoparticle-based intravitreal injection is used for retinal imaging and for the inhibition of retinal neovascularization to treat macular degeneration [78][79][80].
2.2. Nanowires
Engineered nanostructural materials are essential for the development of advanced retinal applications. Among them, nanowires have been reported for retinal applications in recent years [48]. It has also been demonstrated that the structure and morphology of nanowires are similar to those of photoreceptors, and the photoabsorption and charge separation properties of nanowires are comparable to those of photodetectors or solar cells [49]. The gold-nanoparticle-decorated titania (Au-TiO2) nanowire acts as an artificial photoreceptor that restores the light responses in a photoreceptor-degenerated retina. The use of nanowires with poly (ε-caprolactone) (PCL) scaffolds for the delivery of retinal progenitor cells resulted in increased differentiation and migration of these cells into both degenerated and normal retinas [43][50]. Nanowires made of gallium phosphide have been shown to support the long-term survival of photoreceptors (rods and cones), ganglion cells, and bipolar cells [44].
2.3. Hybrid Nanostructures
A vast range of nanomaterials and nanostructures have been explored as neural interfaces in retinal physiology; still, no single material has been successful in mimicking the biological, mechanical, and electrical properties of the retina. In recent years, many hybrid approaches have been designed to explore the merits of many materials while at the same time suppressing their demerits. Recently, Tang et al. demonstrated that artificial photoreceptors made of gold-nanoparticle-decorated titania (Au–TiO2) nanowire arrays were able to absorb light, generate photovoltage, and process visual information in a photoreceptor-degenerated retina [3]. Not only is nanowire arrays’ rough morphology useful for their association with cultured neurons, but they are also biocompatible or (photo)chemically stable for over 2 months when used as a subretinal implant in mice [55]. The use of a gold coating on carbon nanotubes (Au-CNTs) further enhanced their surface area and electrical and mechanical adhesion [85]. Iridium oxide–carbon nanotube hybrids (IrOx-CNT) were reported to have a high effective surface area and much higher charge storage capacities compared to pure iridium oxide [56]. Furthermore, the hybrid coatings formed by combining iridium oxide with reduced graphene oxide or graphene oxide exhibited 10% higher charge storage capacities than those of pure iridium oxide and iridium oxide–carbon nanotube hybrids, indicating superior electrochemical stability [41][57][58][59][60].
2.4. Nanoscaffolds
Nanoscaffolds are self-assembled or electrospun nanofibers made up of synthetic or natural polymers. Nanoscaffolds provide a microenvironment for cellular signaling that influences the proliferation, migration, and differentiation of various cells [86].
3. Studies on the Application of Nano-Biomaterials for Retinal Regeneration
| Analysis | Nanomaterial | Form | Size (nm) | Cell Response | Ref. |
|---|---|---|---|---|---|
| In vitro | Poly (ε-caprolactone) (PCL) | NWs | Length: 2500 |
↑ expression of PKC and recoverin in RPCs; cells undergo differentiation | [43] |
| Gallium phosphide (GaP) | NWs | Length: 500–4000 |
Extended growth of retinal cells | [50] | |
| n-type silicon | NWs | Length: 440 |
Long-term and dense growth of mouse retinal cells | [95] | |
| Gold (Au) | Nanoparticle | Diameter: 5–100 |
ARPE-19 cells undergo apoptosis upon AuNP internalization | [66] | |
| Diameter: 10–12 |
Gold nanoparticles inhibit proliferation of ARPE-19 cells; no cytotoxicity | [5] | |||
| Diameter: 80 |
Highly viable mesenchymal stem cells undergo differentiation and secrete various trophic factors | [4] | |||
| Gold (Au), silver (Ag) |
Nanoparticle | Diameter: 20–80 |
Increase uptake into retinal cells; ↑ apoptosis, oxidative stress, and microglia activation | [47] | |
| Gold (Au) | Nanodisk | Diameter: 160 |
Inhibition of in vitro angiogenesis without cellular toxicity of HRMECs | [45] | |
| Hybrid nanoscaffolds |
Combination of Antheraea pernyi silk fibroin (RWSF), PCL, and gelatin |
Diameter/porosity: 90–210 |
Increased expression of RPE marker genes (CRALBP, PEDF, VEGF, MITF, and PMEL 17 among others) |
[72] | |
| In vivo | Poly (ε-caprolactone) (PCL) membranes | NWs | Length: 2500 |
Successful implantation into subretinal space with limited tissue disruption and no inflammation | [43] |
| Gold (Au), titania (TiO2) | Au nanoparticle coated TiO2 NWs | AuNPs diameter: 5–15, TiO2 NW length: 2000 |
AuNP-decorated TiO2 NW arrays restore light-sensitive visual responses in degenerated photoreceptors | [3] | |
| Gold (Au) | Nanodisk | Diameter: 160 | Intravitreal injection attenuates neovascularization in mouse model of oxygen-induced retinopathy | [45] | |
| Gold (Au) | Nanoparticle | Diameter: 20–100 |
Intravitreal injection of gold nanoparticles passed through the blood–retinal barrier with no structural abnormality or cell death | [80] | |
| Gold (Au) | Nano-gold | Not reported | No retinal or optic nerve toxicity by intravitreal injection of nano-gold | [32][80] | |
| Gold (Au), poly (strenesulfate) | Poly (strenesulfate) or anti-CD90.2 antibody-coated Au nanorods (PSS-AuNRs) | Not reported | Intravitreal injection obscured the retinal signal and induced ocular inflammation | [46] | |
| Nanoscaffolds | Nanofibrous porous membrane | Diameter/porosity: 680 | Bruch’s membrane thickness changes with aging, and it correlates with RPE function | [72] | |
| Therapeutic | Gold (Au) | Nanoparticles | Diameter: 20 |
AuNP-labeled photoreceptor precursor transplantation provides high-resolution long-term tracking and cell survival with no toxic effects on retina or cells | [80][96] |
| Core–shell-structured β-NaYF4:20%Yb, 2%Er@β-NaYF4 | Nanoparticle (core–shell-structured upconversion nanoparticles (UCNPs)) |
Diameter: 35–40 |
Retinal pbUCNP injection extends the visual spectrum to the near infra-red range in mice | [4] | |
| Synthetic nanoscaffolds |
Nanofibrous scaffolds | Diameter/porosity: 100–200 |
Used as a cell replacement therapy | [86] |
This entry is adapted from the peer-reviewed paper 10.3390/nano11081880
References
- Mains, J.; Wilson, C.G. The vitreous humor as a barrier to nanoparticle distribution. J. Ocul. Pharmacol. Ther. 2013, 29, 143–150.
- Mitragotri, S.; Anderson, D.G.; Chen, X.; Chow, E.K.; Ho, D.; Kabanov, A.V.; Karp, J.M.; Kataoka, K.; Mirkin, C.A.; Petrosk, S.H.; et al. Accelerating the translation of nanomaterials in biomedicine. ACS Nano 2015, 9, 6644–6654.
- Tang, J.; Qin, N.; Chong, Y.; Diao, Y.; Yiliguma; Wang, Z.; Xue, T.; Jiang, M.; Zhang, J.; Zheng, G. Nanowire arrays restore vision in blind mice. Nat. Commun. 2018, 9, 786.
- Ma, Y.; Bao, J.; Zhang, Y.; Li, Z.; Zhou, X.; Wan, C.; Huang, L.; Zhao, Y.; Han, G.; Xue, T. Mammalian near-infrared image vision through injectable and self-powered retinal nanoantennae. Cell 2019, 177, 243–255.
- Chen, J.; Patil, S.; Seal, S.; Mcginnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150.
- Liu, X.L.; Chen, S.; Zhang, H.; Zhou, J.; Fan, H.M.; Liang, X.J. Magnetic nanomaterials for advanced regenerative medicine: The promise and challenges. Adv. Mater. 2019, 31, e1804922.
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295.
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742.
- Gao, Y.; Lim, J.; Teoh, S.H.; Xu, C. Emerging translational research on magnetic nanoparticles for regenerative medicine. Chem. Soc. Rev. 2015, 44, 6306–6329.
- Xu, C.; Sun, S. New forms of superparamagnetic nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 732–743.
- Liu, X.L.; Yang, Y.; Ng, C.T.; Zhao, L.Y.; Zhang, Y.; Bay, B.H.; Fan, H.M.; Ding, J. Magnetic Vortex Nanorings: A new class of hyperthermia agent for highly efficient in vivo regression of tumors. Adv. Mater. 2015, 27, 1939–1944.
- Onoshima, D.; Yukawa, H.; Baba, Y. Multifunctional quantum dots-based cancer diagnostics and stem cell therapeutics for regenerative medicine. Adv. Drug Deliv. Rev. 2015, 95, 2–14.
- de Mel, A.; Oh, J.T.; Ramesh, B.; Seifalian, A.M. Biofunctionalized quantum dots for live monitoring of stem cells: Applications in regenerative medicine. Regen Med. 2012, 7, 335–347.
- Zhu, L.; Chang, D.W.; Dai, L.; Hong, Y. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano Lett. 2007, 7, 3592–3597.
- Tran, P.A.; Zhang, L.T.; Webster, A.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114.
- Golovin, Y.I.; Gribanovsky, S.L.; Golovin, D.Y.; Zhigachev, A.O.; Klyachko, N.L.; Majouga, A.G.; Sokolsky, M.; Kabanov, A.V. The dynamics of magnetic nanoparticles exposed to non-heating alternating magnetic field in biochemical applications: Theoretical study. J. Nanopart. Res. 2017, 19, 59.
- Muller, D.J.; Helenius, J.; Alsteens, D.; Dufrene, Y.F. Force probing surfaces of living cells to molecular resolution. Nat. Chem. Biol. 2009, 5, 383–390.
- Wu, C.; Shen, Y.; Chen, M.; Wang, K.; Li, Y.; Cheng, Y. Recent advances in magnetic-nanomaterial-based mechanotransduction for cell fate regulation. Adv. Mater. 2018, 30, e1705673.
- Monzel, C.; Vicario, C.; Piehler, J.; Coppey, M.; Dahan, M. Magnetic control of cellular processes using biofunctional nanoparticles. Chem. Sci. 2017, 8, 7330–7338.
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 2015, 115, 10637–10689.
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem. Rev. 2017, 117, 10121–10211.
- Wu, L.; Garcia, A.M.; Li, Q.; Sun, S. Organic phase syntheses of magnetic nanoparticles and their applications. Chem. Rev. 2016, 116, 10473–10512.
- Noh, S.H.; Na, W.; Jang, J.T.; Lee, J.H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.S.; Cheon, J. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012, 12, 3716–3721.
- Reimer, P.; Balzer, T. Ferucarbotran (Resovist): A new clinically approved res-specific contrast agent for contrast-enhanced MRI of the liver: Properties, clinical development, and applications. Eur. Radiol. 2003, 13, 1266–1276.
- Shu, W.; Wang, Y.; Liu, C.; Li, R.; Pei, C.; Lou, W.; Lin, S.; Di, W.; Wan, J. construction of a plasmonic chip for metabolic analysis in cervical cancer screening and evaluation. Small Methods 2020, 4, 1900469.
- Liu, J.; Cai, C.; Wang, Y.; Liu, Y.; Huang, L.; Tian, T.; Yao, Y.; Wei, J.; Chen, R.; Zhang, K.; et al. A biomimetic plasmonic nanoreactor for reliable metabolite detection. Adv. Sci. 2020, 7, 1903730.
- Hu, D.; Zou, L.; Gao, Y.; Jin, Q.; Ji, J. Emerging nanobiomaterials against bacterial infections in postantibiotic era. View 2020, 1, 20200014.
- Huang, L.; Gurav, D.D.; Wu, S.; Xu, W.; Vedarethinam, V.; Yang, J.; Su, H.; Wan, X.; Fang, Y.; Shen, B.; et al. A multifunctional platinum nanoreactor for point-of-care metabolic analysis. Matter 2019, 1, 1669–1680.
- Yang, J.; Wang, R.; Huang, L.; Zhang, M.; Niu, J.; Bao, C.; Shen, N.; Dai, M.; Guo, Q.; Wang, Q.; et al. Urine metabolic fingerprints encode subtypes of kidney diseases. Angew. Chem. Int. Ed. Engl. 2020, 59, 1703–1710.
- Cao, J.; Shi, X.; Gurav, D.D.; Huang, L.; Su, H.; Li, K.; Niu, J.; Zhang, M.; Wang, Q.; Jiang, M.; et al. Metabolic fingerprinting on synthetic alloys for medulloblastoma diagnosis and radiotherapy evaluation. Adv. Mater. 2020, 32, 2000906.
- Chan, C.M.; Hsiao, C.Y.; Li, H.J.; Fang, J.Y.; Chang, D.C.; Hung, C.F. The inhibitory effects of gold nanoparticles on vegf-a-induced cell migration in choroid-retina endothelial cells. Int. J. Mol. Sci. 2020, 21, 109.
- Bakri, S.J.; Pulido, J.S.; Mukherjee, P.; Marler, R.J.; Mukhopadhyay, D. Absence of histologic retinal of intravitreal nanogold in a rabbit model. Retina 2008, 28, 147–149.
- Kim, J.H.; Kim, J.H.; Kim, K.W.; Kim, M.H.; Yu, Y.S. Intravenously administered gold nanoparticles pass through the blood-retinal barrier depending on the particle size and induce no retinal toxicity. Nanotechnology 2009, 20, 505101.
- Karakocak, B.B.; Raliya, R.; Davis, J.T.; Chavalmane, S.; Wang, W.N.; Ravi, N.; Biswas, P. Biocompatibility of gold nanoparticles in retinal pigment epitheial cell line. Toxicol. Vitro 2016, 37, 61–69.
- Soderstjerna, E.; Bauer, P.; Cedervall, T.; Abdshill, H.; Johansson, F.; Johansson, U.E. Silver and gold nanoparticles exposure to in vitro cultured retina-studies on nanoparticle internalization, apoptosis, oxidative stress, glial- and microglial activity. PLoS ONE 2014, 9, e105359.
- Song, H.B.; Wi, J.S.; Jo, D.H.; Kim, J.H.; Lee, S.W. Intraocular application of gold nanodisks optically tuned for optical coherence tomography: Inhibitory effect on retinal neovascularization without unbearable toxicity. Nanomedicine 2017, 13, 1901–1911.
- Kim, S.J. Novel approaches for retinal drug and gene delivery. Transl. Vis. Sci. Technol. 2014, 3, 7.
- Sheikpranbabu, S.; Kalishwaralal, K.; Venkataraman, D.; Eom, S.H.; Park, J.; Gurunathan, S. Silver nanoparticles inhibit vegf-and il-1β-induced vascular permeability via src dependent pathway in porcine retinal endothelial cells. J. Nanobiotechnol. 2009, 7, 8.
- Maya-Vetencourt., J.F.; Manfredi, G.; Mete, M.; Colombo, E.; Bramini, M.; Di Marco, S.; Shmal, D.; Mantero, G.; Dipalo, M.; Rocchi, A.; et al. Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat. Nanotechnol. 2020, 15, 698–708.
- Sharma, R.; Khristov, V.; Rising, A.; Jha, B.S.; Dejene, R.; Hotaling, N.; Li, Y.; Stoddard, J.; Stankewicz, C.; Wan, Q.; et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci. Transl. Med. 2019, 11, eaat5580.
- Wang, K.; Tang, R.Y.; Zhao, X.B.; Li, J.J.; Lang, Y.R.; Jiang, X.X.; Sun, H.J.; Lin, Q.X.; Wang, C.Y. Covalent bonding of yigsr and rgd to pedot/pss/mwcnt-cooh composite material to improve the neural interface. Nanoscale 2015, 7, 18677–18685.
- Ito, A.; Takizawa, Y.; Honda, H.; Hata, K.I.; Kagami, H.; Ueda, M.; Kobayashi, T. Tissue engineering using magnetite nanoparticles and magnetic force: Heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004, 10, 833–840.
- Redenti, S.; Tao, S.L.; Yang, J.; Gu, P.; Klassen, H.; Saigal, S.; Desai, T.; Young, M.J. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly (e-caprolactone) nanowire scaffold. J. Ocul. Biol. Dis. Inform. 2008, 1, 19–29.
- Piret, G.; Perez, M.T.; Prinz, C.N. Neurite outgrowth and synaptophysin expression of postnatal cns neurons on gap nanowire arrays in long-term retinal cell culture. Biomaterials 2013, 34, 875–887.
- Piret, G.; Perez, M.T.; Prinz, C.N. Substrate porosity induces phenotypic alterations in retinal cells cultured on silicon nanowires. RSC Adv. 2014, 4, 27888–27897.
- Gabriele Sandrian, M.; Wollstein, G.; Schuman, J.S.; Bilonick, R.A.; Ling, Y.; Ishikawa, H.; Kagemann, L.; McKenna, K.C. Inflammatory Response to intravitreal injection of gold nanorods. Br. J. Ophthalmol. 2012, 96, 1522–1529.
- Gurunathan, S.; Lee, K.; Kalishwaralal, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S.H. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009, 30, 6341–6350.
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22.
- Tang, J.; Huo, Z.; Brittman, S.; Gao, H.; Yang, P. Solution-processed core-shell nanowires for efficient photovoltaic cells. Nat. Nanotechnol. 2011, 6, 568–572.
- Christiansen, A.T.; Tao, S.L.; Smith, M.; Wnek, G.E.; Prause, J.U.; Young, M.J.; Klassen, H.; Kaplan, H.J.; la Cour, M.; Kiilgaard, J.F. Subretinal implantation of electrospun, short nanowire, and smooth poly (ε-caprolactone) scaffolds to the subretinal space of porcine eyes. Stem Cells Int. 2012, 2012, 454295.
- Kang, M.; Lee, H.; Kang, T.; Kim, B. Synthesis, properties, and biological application of perfect crystal gold nanowires: A review. J. Mater. Sci. Technol. 2015, 31, 573–580.
- Lee, S.; Jung, S.W.; Ahn, J.; Yoo, H.J.; Oh, S.J.; Cho, D.D. Microelectrode array with integrated nanowire fet switches for high-resolution retinal prosthetic systems. J. Micromech. Microeng. 2014, 24, 075018.
- SanMartin, A.; Johansson, F.; Samuelson, L.; Prinz, C.N. Microarray analysis reveals moderate gene expression changes in cortical neural stem cells cultured on nanowire arrays. J. Nanosci. Nanotechnol. 2014, 14, 4880–4885.
- Gautam, V.; Rand, D.; Hanein, Y.; Narayan, K. A polymer optoelectronic interface provides visual cues to a blind retina. Adv. Mater. 2014, 26, 1751–1756.
- Parameswaran, R.; Carvalho-de- Souza, J.L.; Jiang, Y.; Burke, M.J.; Zimmerman, J.F.; Koehler, K.; Phillips, A.W.; Yi, J.; Adams, E.J.; Bezanilla, F.; et al. Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat. Nanotechnol. 2018, 13, 260–266.
- Vafaiee, M.; Mohammadpour, R.; Vossoughi, M.; Elham Asadian, E.; Janahmadi, M.; Sasanpour, P. Carbon nanotube modified microelectrode array for neural interface. Front. Bioeng. Biotechnol. 2021, 8, 582713.
- Carretero, N.M.; Lichtenstein, M.P.; Pérez, E.; Cabana, L.; Suñol, C.; Casañ-Pastor, N. IrOx–carbon nanotube hybrids: A nanostructured material for electrodes with increased charge capacity in neural systems. Acta Biomater. 2014, 10, 4548–4558.
- Pérez, E.; Lichtenstein, M.; Suñol, C.; Casañ-Pastor, N. Coatings of nanostructured pristine graphene-irox hybrids for neural electrodes: Layered stacking and the role of non-oxygenated graphene. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 218–226.
- Carretero, N.M.; Lichtenstein, M.; Pérez, E.; Sandoval, S.; Tobias, G.; Suñol, C.; Casan-Pastor, N. Enhanced charge capacity in iridium oxide-graphene oxide hybrids. Electrochim. Acta 2015, 157, 369–377.
- Xiao, H.; Zhang, M.; Xiao, Y.; Che, J. A feasible way for the fabrication of single walled carbon nanotube/polypyrrole composite film with controlled pore size for neural interface. Colloids Surf. B Biointerfaces 2015, 126, 138–145.
- Zhou, H.; Cheng, X.; Rao, L.; Li, T.; Duan, Y.Y. Poly (3, 4-ethylenedioxythiophene)/multiwall carbon nanotube composite coatings for improving the stability of microelectrodes in neural prostheses applications. Acta Biomater. 2013, 9, 6439–6449.
- Rose, J.B.; Pacelli, S.; Haj, A.J.E.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R.A.J. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135.
- White, C.E.; Olabisi, R.M. Scaffolds for retinal pigment epithelial cell transplantation in age-related macular degeneration. J. Tissue Eng. 2017, 8.
- Noorani, B.; Tabandeh, F.; Yazdian, F.; Soheili, Z.-S.; Shakibaie, M.; Rahmani, S. Thin natural gelatin/chitosan nanofibrous scaffolds for retinal pigment epithelium cells. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 754–763.
- Karamichos, D. Ocular Tissue Engineering: Current and Future Directions. J. Funct. Biomater. 2015, 6, 77–80.
- Hynes, S.R.; Lavik, E.B. A tissue-engineered approach towards retinal repair: Scaffolds for cell transplantation to the subretinal space. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 763–778.
- Pritchard, C.D.; Arnér, K.M.; Neal, R.A.; Neeley, W.L.; Bojo, P.; Bachelder, E.; Holz, J.; Watson, N.; Botchwey, E.A.; Langer, R.S.; et al. The use of surface modified poly (glycerol-co-sebacic acid) in retinal transplantation. Biomaterials 2010, 31, 2153–2162.
- Warnke, P.H.; Alamein, M.; Skabo, S.; Stephens, S.; Bourke, R.; Heiner, P.; Liu, Q. Primordium of an artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater. 2013, 9, 9414–9422.
- McHugh, K.J.; Tao, S.L.; Saint-Geniez, M. Porous poly(ε-caprolactone) scaffolds for retinal pigment epithelium transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1754–1762.
- Giordano, G.G.; Thomson, R.C.; Ishaug, S.L.; Mikos, A.G.; Cumber, S.; Garcia, C.A.; Lahiri-Munir, D. Retinal pigment epithelium cells cultured on synthetic biodegradable polymers. J. Biomed. Mater. Res. 1997, 34, 87–93.
- Ilmarinen, T.; Hiidenmaa, H.; Kööbi, P.; Nymark, S.; Sorkio, A.; Wang, J.H.; Stanzel, B.V.; Thieltges, F.; Alajuuma, P.; Oksala, O.; et al. Ultrathin Polyimide Membrane as Cell Carrier for Subretinal Transplantation of Human Embryonic Stem Cell Derived Retinal Pigment Epithelium. PLoS ONE 2015, 10, e0143669.
- Xiang, P.; Wu, K.C.; Zhu, Y.; Xiang, L.; Li, C.; Chen, D.L.; Chen, F.; Xu, G.; Wang, A.; Li, M.; et al. A novel Bruch’s membrane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells. Biomaterials 2014, 35, 9777–9788.
- Kim, J.H.; Kim, M.H.; Jo, D.H.; Yu, Y.S.; Lee, T.J.; Kim, J.H. The inhibition of retinal neovascularization by gold nanoparticles via suppression of vegfr-2 activation. Biomaterials 2011, 32, 1865–1871.
- Joris, F.; Manshian, B.B.; Peynshaert, K.; De Smedt, S.C.; Braeckmans, K.; Soenen, S.J. Assessing nanoparticle toxicity in cell-based assays: Influence of cell culture parameters and optimized models for bridging the in vitro-in vivo gap. Chem. Soc. Rev. 2013, 42, 8339–8359.
- Li, Y.; Yue, T.T.; Yang, K.; Zhang, X.R. Molecular modeling of the relationship between nanoparticle shape anisotropy and endocytosis kinetics. Biomaterials 2012, 33, 4965–4973.
- Ngwa, W.; Makrigiorgos, G.M.; Berbeco, R. Gold nanoparticle enhancement of stereotactic radiosurgery for neovascular age-related macular degeneration. Phys. Med. Biol. 2012, 57, 6371–6380.
- Ngwa, W.; Makrigiorgos, G.M.; Berbeco, R. SU-E-T-408: Enhancing stereotactic radiosurgery for neovascular age-related macular degeneration, using gold nanoparticles. Med. Phys. 2012, 39, 3798.
- Farjo, K.M.; Ma, J.X. The potential of nanomedicine therapies to treat neovascular disease in the retina. J. Angiogenes Res. 2010, 2, 21.
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Prog. Retin. Eye Res. 2010, 29, 596–609.
- Hayashi, A.; Naseri, A.; Pennesi, M.E.; de Juan, E., Jr. Subretinal delivery of immunoglobulin g with gold nanoparticles in the rabbit eye. Jpn. J. Ophthalmol. 2009, 53, 249–256.
- De Matteis, V.; Cascione, M.; Cristina, C.; Rinaldi, R. Engineered gold nanoshells killing tumor cells: New perspectives. Curr. Pharm. Des. 2019, 25, 1477–1489.
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317.
- Loudin, J.; Simanovskii, D.; Vijayraghavan, K.; Sramek, C.; Butterwick, A.; Huie, P.; Mclean, G.Y.; Palanker, D.V. Optoelectronic retinal prosthesis: System design and performance. J. Neural Eng. 2007, 4, S72–S84.
- Sealy, C. Nanowires promise new ways to restore vision and movement. Nano Today 2018, 20, 1–2.
- Chinh, V.D.; Speranza, G.; Migliaresi, C.; Van Chuc, N.; Tan, V.M.; Phuong, N.T. Synthesis of gold nanoparticles decorated with multiwalled carbon nanotubes (Au-MWCNTs) via cysteaminium chloride functionalization. Sci. Rep. 2019, 9, 5667.
- Hotaling, N.A.; Khristov, V.; Wan, Q.; Sharma, R.; Jha, B.S.; Lotfi, M.; Maminishkis, A.; Simon, C.G., Jr.; Bharti, K. Nanofiber scaffold-based tissue-engineered retinal pigment epithelium to treat degenerative eye diseases. J. Ocul. Pharmacol. Ther. 2016, 32, 272–285.
- Santos-Ferreira, T.; Llonch, S.; Borsch, O.; Postel, K.; Haas, J.; Ader, M. Retinal transplantation of photoreceptors results in donor–host cytoplasmic exchange. Nat. Commun. 2016, 7, 13028.
- Biazar, E.; Baradaran-Rafii, A.; Heidari-keshel, S.; Tavakolifard, S. Oriented nanofibrous silk as a natural scaffold for ocular epithelial regeneration. J. Biomater. Sci. Polym. Ed. 2015, 26, 1139–1151.
- Komez, A.; Baran, E.T.; Erdem, U.; Hasirci, N.; Hasirci, V. Construction of a patterned hydrogel-fibrous mat bilayer structure to mimic choroid and bruch’s membrane layers of retina. J. Biomater. Res. A 2016, 104, 2166–2177.
- Shrestha, B.K.; Shrestha, S.; Baral, E.R.; Lee, J.Y.; Kim, B.S.; Park, C.H.; Kim, C.S. π-Conjugated polyaniline-assisted flexible titania nanotubes with controlled surface morphology as regenerative medicine in nerve cell growth. Chem. Eng. J. 2019, 360, 701–713.
- Jaggessar, A.; Mathew, A.; Wang, H.; Tesfamichael, T.; Yan, C.; Yarlagadda, P.K. Mechanical, bactericidal and osteogenic behaviours of hydrothermally synthesised tio2 nanowire arrays. J. Mech. Behav. Biomed. Mater. 2018, 80, 311–319.
- Lin, H.I.; Kuo, S.W.; Yen, T.J.; Lee, O.K. Si NWs biophysically regulate the fates of human mesenchymal stem cells. Sci. Rep. 2018, 8, 12913.
- Li, Z.; Persson, H.; Adolfsson, K.; Oredsson, S.; Prinz, C.N. Morphology of living cells cultured on nanowire arrays with varying nanowire densities and diameters. Sci. China Life Sci. 2018, 61, 427–435.
- Masse, F.; Ouellette, M.; Lamoureux, G.; Boisselier, E. Gold nanoparticles in ophthalmology. Med. Res. Rev. 2019, 39, 302–327.
- Wang, R.; Huang, X.; Liu, G.; Wang, W.; Dong, F.; Li, Z. Fabrication and characterization of a parylene-based three-dimensional microelectrode array for use in retinal prosthesis. J. Microelectromech. Syst. 2010, 19, 367–374.
- Chemla, Y.; Betzer, O.; Markus, A.; Farah, N.; Motiei, M. Gold nanoparticles for multimodal high-resolution imaging of transplanted cells for retinal replacement therapy. Nanomedicine 2019, 14, 1857–1871.
