Salt-tolerant plant growth-promoting rhizobacteria (PGPR) could be an alternative to
alleviate salinity problems in rice plants grown in the coastal areas. This study was conducted
to isolate and characterize salt-tolerant PGPR and observe their effects on the physiological and
biochemical properties of rice plants grown under non-saline and saline glasshouse conditions.
Three strains were selected based on their salt-tolerance and plant growth-promoting properties under
in vitro saline conditions. These strains were identified as Bacillus tequilensis (UPMRB9), Bacillus
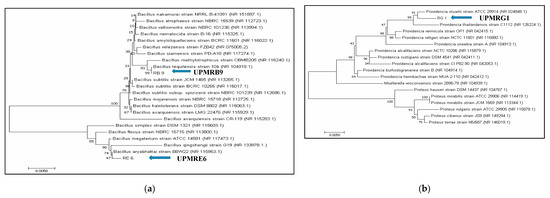
aryabhattai (UPMRE6), and Providencia stuartii (UPMRG1) using a 16S rRNA technique. The selected
strains were inoculated to three different rice varieties, namely BRRI dhan67 (salt-tolerant), Putra-1
(moderate salt-tolerant), and MR297 (salt-susceptible) under glasshouse conditions. Results showed
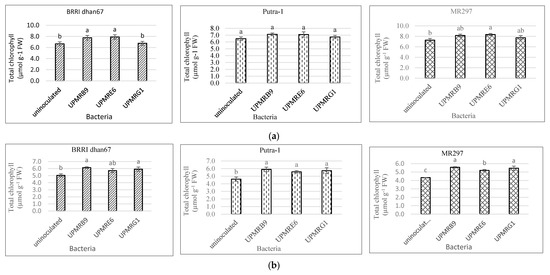
that the MR297 rice variety inoculated with UPMRB9 produced the highest total chlorophyll content,
with an increment of 28%, and the lowest electrolyte leakage of 92%. The Putra-1 rice variety also showed
a 156% total dry matter increase with the inoculation of this bacterial strain. The highest increase of
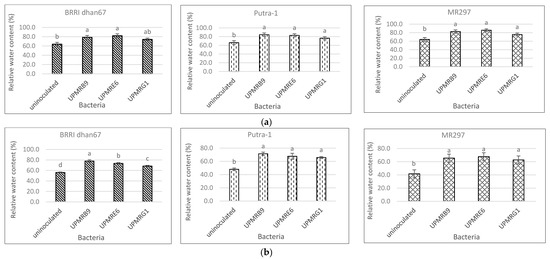
relative water content and reduction of Na/K ratio were found upon inoculation of UPMRE6 and
UPMRB9, respectively. The biggest significant effects of these bacterial inoculations were on relative
water content, electrolyte leakage, and the Na/K ratio of the BRRI dhan67 rice variety under saline
conditions, suggesting a synergistic effect on the mechanisms of plant salt-tolerance. This study
has shown that the application of locally-isolated salt-tolerant PGPR strains could be an effective
long-term and sustainable solution for rice cultivation in the coastal areas, which are affected by
global climate change.
- PGPR
- salt-tolerant
- rice
- salinity
- climate change
- Definition
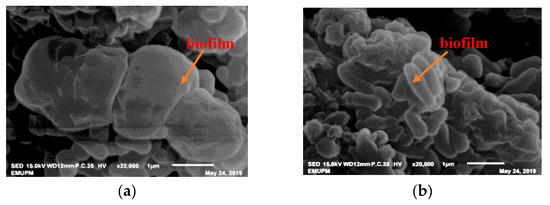
Salt-tolerant Plant Growth-Promoting Rhizobacteria (PGPR) has been reported to possess various salt-tolerant mechanisms, namely through the production of exopolysaccharide (EPS) and the formation of a biofilm, which have been proven to restrict Na+ uptake under saline soil conditions.
- Introduction
- Data, Model, Applications and Influences
3.1. Salt-Tolerance and Plant Growth-Promoting Properties of Selected Bacterial Isolates
| Bacterial Isolates | Salt-Tolerance Characteristics | Plant Growth-Promoting Characteristics | ||||||
| EPS (g L−1) | Floc Yield (g L−1) | Biofilm (590nm) | Sodium Uptake (mg L−1) | IAA (µg mL−1) | P Solubilization (µg mL−1) | K Solubilization (mg L−1) | Nitrogen Fixation | |
| UPMRA4 | 7.36c | 17.83a | 0.44d | 7.55cd | 6.71b | 9.21b | 2.08ab | − |
| UPMRB9 | 30.50a | 19.28a | 1.14a | 23.8a | 8.25a | 15.18a | 2.17ab | + |
| UPMRE3 | 12.24c | 9.73c | 0.31e | 6.40d | 3.50c | 9.34b | 0.70c | − |
| UPMRE6 | 26.18ab | 19.67a | 0.95b | 12.49bc | 6.70b | 15.30a | 2.97a | + |
| UPMRG1 | 21.79b | 12.90b | 0.77c | 13.51b | 6.99ab | 9.24b | 1.72bc | + |
|
Note: ‘+’ indicates positive, ‘−’ indicates negative. Means with the same letter in a column do not differ significantly using Tukey’s test at p > 0.05. EPS: exopolysaccharide; IAA: indole-3-acetic acid. |
||||||||
3.2. Identification of PGPR Isolates Based on Partial 16S rRNA Gene Sequences
3.3. Effect of Bacterial Inoculation on Total Chlorophyll Content of Three Rice Varieties
3.4. Effect of Bacterial Inoculation on the Relative Water Content of Three Rice Varieties
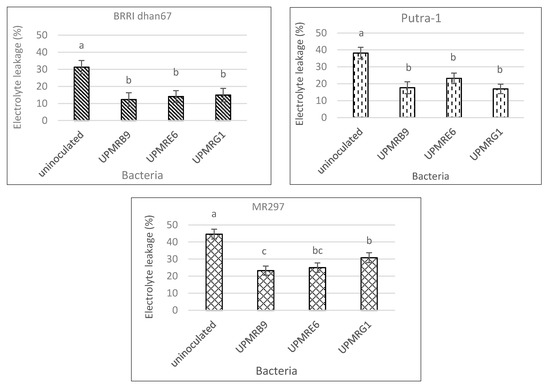
3.5. Effect of Bacterial Inoculation on Electrolyte Leakage of Three Rice Varieties
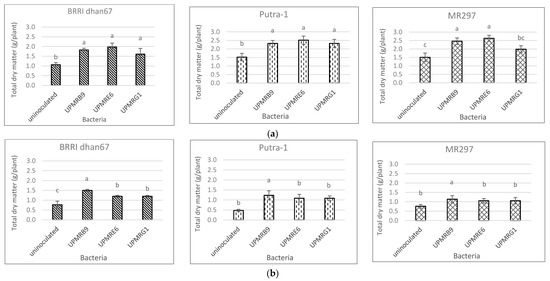
3.6. Effect of Bacterial Inoculations on Total Dry Matter Production of Three Rice Varieties
3.7. Effect of Salinity, PGPR and Rice Varieties on Na/K Ratio in the Above-Ground (Shoot) and Below Ground (Root and Soil) Parts of Three Rice Varieties
| Variety | Shoot | |||
| Uninoculated | UPMRB9 | UPMRE6 | UPMRG1 | |
| BRRI dhan67 | 0.73a | 0.14c (81%) | 0.22b (70%) | 0.26b (64%) |
| Putra-1 | 1.13a | 0.39c (66%) | 0.57b (50%) | 0.60b (47%) |
| MR297 | 1.37a | 0.81c (41%) | 1.04b (24%) | 1.09b (20%) |
|
Means having the same letter in each variety do not differ significantly using Tukey’s test at p > 0.05. |
||||
| Variety | Root | Soil | ||||||
| CONTROL | UPMRB9 | UPMRE6 | UPMRG1 | CONTROL | UPMRB9 | UPMRE6 | UPMRG1 | |
| BRRI dhan67 | 0.61a | 0.29c (53%) | 0.50b (18%) | 0.53b (13%) | 0.50b | 0.81a (62%) | 0.67ab (34%) | 0.63b (26%) |
| Putra-1 | 0.79a | 0.90a (14%) | 0.42b (47%) | 0.45b (43%) | 0.46b | 0.64a (39%) | 0.48b (4%) | 0.46b (0%) |
| MR297 | 1.11a | 1.04a (6%) | 0.60b (46%) | 0.56b (50%) | 0.27b | 0.59a (119%) | 0.30b (11%) | 0.37b (37%) |
|
Means having the same letter in each variety do not differ significantly using Tukey’s test at p > 0.05. |
||||||||
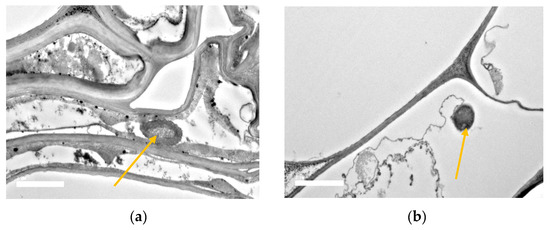
3.8. SEM and TEM Observations of Bacterial Root Colonization

This entry is adapted from the peer-reviewed paper 10.3390/agronomy10071030
References
- I. Nemati; Foad Moradi; S. Gholizadeh; M.A. Esmaeili; M.R. Bihamta; The effect of salinity stress on ions and soluble sugars distribution in leaves, leaf sheaths and roots of rice (Oryza sativa L.) seedlings. Plant, Soil and Environment 2011, 57, 26-33, 10.17221/71/2010-pse.
- Samant A, Jawali N; Early seedling stage salt tolerance evaluation of genetically diverse rice genotypes. Ann. Biol. Res. 2016, 7, 46-54, .
- Bhaswati Ghosh; Nasim Ali; Response of Rice under Salinity Stress: A Review Update. Rice Research: Open Access 2016, 4, 1-8, 10.4172/2375-4338.1000167.
- S Viscardi; V Ventorino; P Duran; A Maggio; Stefania De Pascale; M.L Mora; O Pepe; Assessment of plant growth promoting activities and abiotic stress tolerance of Azotobacter chroococcum strains for a potential use in sustainable agriculture. Journal of Soil Science and Plant Nutrition 2016, 16, 848-863, 10.4067/S0718-95162016005000060.
- Bhavanath Jha; Iti Gontia; Anton Hartmann; The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential. Plant and Soil 2011, 356, 265-277, 10.1007/s11104-011-0877-9.
- Salme Timmusk; Seong-Bin Kim; Eviatar Nevo; Islam Abd El Daim; Bo Ek; Jonas Bergquist; Lawrence Behers; Sfp-type PPTase inactivation promotes bacterial biofilm formation and ability to enhance wheat drought tolerance. Frontiers in Microbiology 2015, 6, 387, 10.3389/fmicb.2015.00387.
- Mahwish Zahid; M. Kaleem Abbasi; Sohail Hameed; Nasir Rahim; Isolation and identification of indigenous plant growth promoting rhizobacteria from Himalayan region of Kashmir and their effect on improving growth and nutrient contents of maize (Zea mays L.). Frontiers in Cellular and Infection Microbiology 2015, 6, 207, 10.3389/fmicb.2015.00207.
- Afshan Majeed; M. Kaleem Abbasi; Sohail Hameed; Asma Imran; Nasir Rahim; Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Frontiers in Cellular and Infection Microbiology 2015, 6, 198, 10.3389/fmicb.2015.00198.
- John M. Whipps; Microbial interactions and biocontrol in the rhizosphere. Journal Of Experimental Botany 2001, 52, 487-511, 10.1093/jexbot/52.suppl_1.487.
- Shimon Mayak; Tsipora Tirosh; Bernard R Glick; Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiology and Biochemistry 2004, 42, 565-572, 10.1016/j.plaphy.2004.05.009.
- D. Saravanakumar; R. Samiyappan; ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. Journal of Applied Microbiology 2007, 102, 1283-1292, 10.1111/j.1365-2672.2006.03179.x.
- Pushp Sheel Shukla; Pradeep K. Agarwal; Bhavanath Jha; Improved Salinity Tolerance of Arachis hypogaea (L.) by the Interaction of Halotolerant Plant-Growth-Promoting Rhizobacteria. Journal of Plant Growth Regulation 2011, 31, 195-206, 10.1007/s00344-011-9231-y.
- Himadri Bhusan Bal; Lipika Nayak; Subhasis Das; Tapan K. Adhya; Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant and Soil 2012, 366, 93-105, 10.1007/s11104-012-1402-5.
- S. K. Upadhyay; D. P. Singh; Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biology 2014, 17, 288-293, 10.1111/plb.12173.
- Han HS, Lee KD; Plant growth-promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res. J. Agric. Biol. Sci. 2005, 1, 210-215, .
- M. Rajkumar; R. Nagendran; Kui Jae Lee; Wang Hyu Lee; Sung Zoo Kim; Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 2006, 62, 741-748, 10.1016/j.chemosphere.2005.04.117.
- Mohammad Saghir Khan; Almas Zaidi; Parvaze A. Wani; Role of phosphate-solubilizing microorganisms in sustainable agriculture — A review. Agronomy for Sustainable Development 2007, 27, 29-43, 10.1051/agro:2006011.
- Haiming Liu; Yanjing He; Haixia Jiang; Huasong Peng; Xian-Qing Huang; Xuehong Zhang; Linda S. Thomashow; Yuquan Xu; Characterization of a Phenazine-Producing Strain Pseudomonas chlororaphis GP72 with Broad-Spectrum Antifungal Activity from Green Pepper Rhizosphere. Current Microbiology 2007, 54, 302-306, 10.1007/s00284-006-0444-4.
- D. Saravanakumar; Charles Vijayakumar; N. Kumar; R. Samiyappan; PGPR-induced defense responses in the tea plant against blister blight disease. Crop Protection 2007, 26, 556-565, 10.1016/j.cropro.2006.05.007.
- Parvaze Ahmad Wani; Mohammad Saghir Khan; Almas Zaidi; Effect of metal tolerant plant growth promoting Bradyrhizobium sp. (vigna) on growth, symbiosis, seed yield and metal uptake by greengram plants. Chemosphere 2007, 70, 36-45, 10.1016/j.chemosphere.2007.07.028.
- Aisha Waheed Qurashi; Anjum Nasim Sabri; Biofilm formation in moderately halophilic bacteria is influenced by varying salinity levels. Journal of Basic Microbiology 2011, 52, 566-572, 10.1002/jobm.201100253.
- Yun Chen; Fang Yan; Yunrong Chai; Hongxia Liu; Roberto Kolter; Richard Losick; Jianhua Guo; Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation.. Environmental Microbiology 2012, 15, 848-864, 10.1111/j.1462-2920.2012.02860.x.
- Gholami A, Shahsavani S, Nezarat S; The effect of plant growth promoting rhizobacteria on seed germination and seedling growth of maize. Int. J. Biol. Sci. 2009, 1, 35-40, .
- Yoshida S.. Fundamentals of Rice Crop Production; IRRI:: Los Banos, Philippines, 1981; pp. 1.
