An effective analytical technique for biomass characterisation is inevitable for biomass utilisation in energy production. To improve biomass processing, various thermal conversion methods such as torrefaction, pyrolysis, combustion, hydrothermal liquefaction, and gasification have been widely used to improve biomass processing. Thermogravimetric analysers (TG) and gas chromatography (GC) are among the most fundamental analytical techniques utilised in biomass thermal analysis. Thus, GC and TG, in combination with MS, FTIR, or two-dimensional analysis, were used to examine the key parameters of biomass feedstock and increase the productivity of energy crops. We can also determine the optimal ratio for combining two separate biomass or coals during co-pyrolysis and co-gasification to achieve the best synergetic relationship.
- renewable energy
- thermochemical conversion
- thermal analysis
- thermogravimetric analysers
- gas chromatograph
- biomass and sustainability
1. Introduction
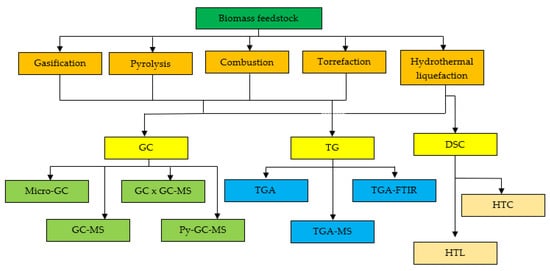
2. Thermochemical Conversion Process
2.1. Gasification
x=w0−wtw0−wf×100%(1)
where w0 is the sample’s initial mass, wt is the sample’s mass over time, and wf is the sample’s final mass. A well-established data analysis approach was used to investigate the reaction mechanism involved in a heat conversion process. The general response rate (dx/dt) is considered to be a function of the conversion (x) and a rate constant (k) and it can be written as Equation (2).
dxdt=kf (x)(2)
The plausible model of the reaction is denoted by f(x), The Arrhenius equation could be used to define the temperature-dependent gasification reaction rate constant (k) as represented in Equation (3).
k = A exp (−ERT)(3)
where A is the pre-exponential factor (min−1), E is the activation energy (kJ/mol), T is the absolute temperature (K), and R is the gas constant (8.314 J/(mol·K) [28]. To recover additional energy, most commercial gasification plants that handle municipal solid waste-derived feedstock use a secondary combustion chamber to burn the syngas and recover energy from a steam circuit. Another important by-product of gasification is solid leftovers of non-combustible materials containing a modest quantity of carbon. At various phases of the gasification process, high-temperature plasma gasification methods are also used. This plasma technology can generate tar-free, pure syngas, and the ash may be fused into glassy or vitreous residue [29]. The energetic efficiency (ηex) is the performance criterion used in the process performance condition. It is defined as the proportion of lucrative energy outputs flowing from the gasifier to the necessary energy input flow [30]:
ηex=£xgas+£xloss+£xtar+£xchar£xbiomass+£xsteam(4)
where £xgas, £xtar, £xchar, £xbiomass, and £xsteam are the loss energy flow and energy flow of gas, tar, char, biomass, and steam, respectively. Entropy creation, heat and mass transfers, and irreversibility of chemical reactions all result in a loss. The first and second thermodynamic laws must be followed in the gasification process. As a result of the second law, it is obtained by the following expression:
∑R£x−∑P£x=I(5)
where £x denotes energy and I denotes irreversibility, and it denotes the internal energy lost as a result of material quality deterioration and energy dissipation [31].
2.2. Pyrolysis
dadt=k (T) f (a)=A exp (−ERT) f (a)(6)
where f (a) is the mechanism function equation, A is the preexponential factor (s−1), E is the activation energy (kJ/mol), and R is the universal gas constant (J/mol·K), a is the reaction conversion degree, t is the time (min) and T is the temperature (K). The value a is obtained by solving the following Equation (5).
a=wi−wwi−wf (7)
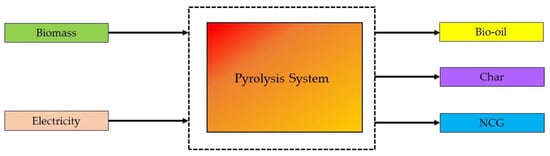
2.3. Combustion
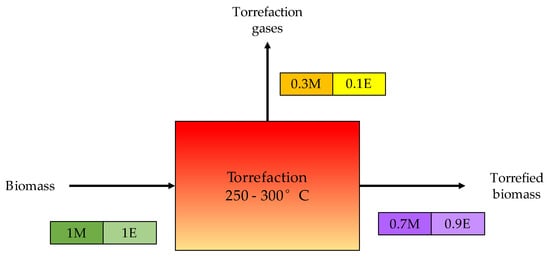
2.4. Torrefaction
2.5. Hydrothermal Liquefaction
2.6. Comparison of Thermal Technologies
Table 1 shows the comparison of five typical thermochemical processes. As can be seen, each of the given processes is subjected to a range of temperatures and pressures for the conversion and to obtain the desired results.
Table 1. Comparison of five thermochemical conversion processes.
| Thermochemical Process | Temperature (°C) | Pressure (MPa) | Gas Products | Pollutants | Purpose | Advantages |
|---|---|---|---|---|---|---|
| Gasification | 500 to 1300 | ≥0.1 | CO2, H2, CO2, H2O, and CH4 | H2S, NH3, tar, and dust | Converting biomass to high HV gas | Production of a wide range of chemical products and the ability to adapt to changing market conditions. |
| Pyrolysis | 300 to 1000 | 0.1 to 0.5 | CO, H2, CH4, and other hydrocarbons | H2S, NH3, tar, and dust | Converting biomass to biochar and bio-oil | Liquid fuels are produced directly, and after appropriate treatment, it can be directly treated in conventional refineries. |
| Combustion | 700 to 1000 | ≥0.1 | CO2 and H2O | SOxy, NOxy, polycyclic aromatic hydrocarbons (PAHs), and dust | Converting biomass to heat and electricity | The procedure is straightforward. Co-combustion of biomass and coal does not necessitate any changes to existing power plants. |
| Torrefaction | 200 to 300 | ≥0.1 | CO2, CO, and CH4 | H2S, COS, CS2, NH3, and HCN | Converting biomass into coal-like material | Moisture reduction, energy density increase, O/C ratio reduction, and improved ignitability and reactivity of the processed fuel. |
| Hydrothermal liquefaction | 250 to 550 | 5 to 25 | H2, CO, CO2, and CH4 | Polypropylene (PE), polypropene (PP), and nylon-6 (NY) | Converting wet biomass into crude-like oil | Process is environmentally friendly. The energy efficiency of the HTL process is very high. |
The most common thermochemical reactions are combustion, torrefaction, pyrolysis, liquefaction, and gasification. When comparing pyrolysis and gasification, the pyrolysis process has a lower reaction temperature than the gasification process. The material’s volatile components are thermally decomposed into more syngas and non-volatile carbon char, which are by-products of the pyrolysis process. The drawback of torrefaction is that as the residence time increases, the hydrogen and oxygen composition of biomass decreases in contrast to carbon, resulting in a decrease in biomass volatile matter content. The liquefaction method has received a lot of attention for utilising biomass waste because of its flexibility and potential to be used as a construction medium for a final product that incorporates all of the positive functional groups present in the liquefying solvents and biomass. Compared to other thermochemical processes, liquefaction requires lower temperatures, allowing it to save more fuel, generate fewer pollutants, and be considerably less expensive.
This entry is adapted from the peer-reviewed paper 10.3390/pr9091610
References
- Chan, Y.H.; Cheah, K.W.; How, B.S.; Loy, A.C.M.; Shahbaz, M.; Singh, H.K.G.; Yusuf, N.R.; Shuhaili, A.F.A.; Yusup, S.; Ghani, W.A.W.A.K.; et al. An overview of biomass thermochemical conversion technologies in Malaysia. Sci. Total Environ. 2019, 680, 105–123.
- Ong, H.C.; Chen, W.H.; Singh, Y.; Gan, Y.Y.; Chen, C.Y.; Show, P.L. A state-of-the-art review on thermochemical conversion of biomass for biofuel production: A TG-FTIR approach. Energy Convers. Manag. 2020, 209, 112634.
- Abdulrazik, A.; Elsholkami, M.; Elkamel, A.; Simon, L. Multi-products productions from Malaysian oil palm empty fruit bunch (EFB): Analyzing economic potentials from the optimal biomass supply chain. J. Clean. Prod. 2017, 168, 131–148.
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 343–362.
- Katsaros, G.; Pandey, D.S.; Horvat, A.; Aranda Almansa, G.; Fryda, L.E.; Leahy, J.J.; Tassou, S.A. Experimental investigation of poultry litter gasification and co-gasification with beech wood in a bubbling fluidised bed reactor—Effect of equivalence ratio on process performance and tar evolution. Fuel 2020, 262, 116660.
- Phillips, D.; Mitchell, E.J.S.; Lea-Langton, A.R.; Parmar, K.R.; Jones, J.M.; Williams, A. The use of conservation biomass feedstocks as potential bioenergy resources in the United Kingdom. Bioresour. Technol. 2016, 212, 271–279.
- Boerrigter, H.; Rauch, R. Review of applications of gases from biomass gasification. ECN Biomass Coal Environ. 2006, 20, 33.
- Zhang, Y.; Zhao, Y.; Gao, X.; Li, B.; Huang, J. Energy and exergy analyses of syngas produced from rice husk gasification in an entrained flow reactor. J. Clean. Prod. 2015, 95, 273–280.
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109546.
- Ozturk, M.; Dincer, I. Integrated Gasification Combined Cycles; Comprehensive Energy System: Oshawa, ON, Canada, 2018; pp. 364–473.
- Kamble, A.D.; Saxena, V.K.; Chavan, P.D.; Mendhe, V.A. Co-gasification of coal and biomass an emerging clean energy technology: Status and prospects of development in Indian context. Int. J. Min. Sci. Technol. 2019, 29, 171–186.
- Guan, G.; Kaewpanha, M.; Hao, X.; Abudula, A. Catalytic steam reforming of biomass tar: Prospects and challenges. Renew. Sustain. Energy Rev. 2016, 58, 450–461.
- Ren, J.; Cao, J.P.; Yang, F.L.; Zhao, X.Y.; Tang, W.; Cui, X.; Chen, Q.; Wei, X.Y. Layered uniformly delocalized electronic structure of carbon supported Ni catalyst for catalytic reforming of toluene and biomass tar. Energy Convers. Manag. 2019, 183, 182–192.
- Liu, N.A.; Fan, W.; Dobashi, R.; Huang, L. Kinetic modeling of thermal decomposition of natural cellulosic materials in air atmosphere. J. Anal. Appl. Pyrolysis 2002, 63, 303–325.
- Xu, C.; Hu, S.; Xiang, J.; Zhang, L.; Sun, L.; Shuai, C.; Chen, Q.; He, L.; Edreis, E.M.A. Interaction and kinetic analysis for coal and biomass co-gasification by TG-FTIR. Bioresour. Technol. 2014, 154, 313–321.
- Seo, Y.-C.; Alam, M.T.; Yang, W.-S. Gasification of Municipal Solid Waste. Gasif. Low Grade Feed. 2018.
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Thermodynamics of gas-char reactions: First and second law analysis. Chem. Eng. Sci. 2003, 58, 1003–1011.
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608.
- Novak, J.M.; Johnson, M.G. Elemental and Spectroscopic Characterization of Low-Temperature (350 °C) Lignocellulosic-and Manure-based Designer Biochars and Their Use as Soil Amendments; Elsevier Inc.: Amsterdam, The Netherlands, 2018.
- Sebastiani, A.; Macrì, D.; Gallucci, K.; Materazzi, M. Steam—oxygen gasification of refuse derived fuel in fluidized beds: Modelling and pilot plant testing. Fuel Process. Technol. 2021, 216, 106783.
- Zhang, Y.; Chen, P.; Liu, S.; Peng, P.; Min, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Fan, L.; Liu, C.; et al. Effects of feedstock characteristics on microwave-assisted pyrolysis—A review. Bioresour. Technol. 2017, 230, 143–151.
- Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; Stefanov, S.; Andonova, V. We are IntechOpen, the World’s Leading Publisher of Open Access Books Built by Scientists, for Scientists TOP 1%; IntechOpen: London, UK, 2016.
- Abnisa, F.; Wan Daud, W.M.A. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85.
- Bhoi, P.R.; Ouedraogo, A.S.; Soloiu, V.; Quirino, R. Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew. Sustain. Energy Rev. 2020, 121, 109676.
- Wu, Z.; Luo, H. Pyrolysis Characteristics and Kinetic Analysis of Sediment from the Dianchi Lake in China. Int. J. Chem. Eng. 2018, 2018.
- Boateng, A.A.; Mullen, C.A.; Osgood-Jacobs, L.; Carlson, P.; Macken, N. Mass Balance, Energy, and Exergy Analysis of Bio-Oil Production by Fast Pyrolysis. J. Energy Resour. Technol. 2012, 134, 042001.
- Grigiante, M.; Ischia, M.; Baratieri, M.; Maschio, R.D.; Ragazzi, M. Pyrolysis analysis and solid residue stabilization of polymers, waste tyres, spruce sawdust and sewage sludge. Waste Biomass Valorization 2010, 1, 381–393.
- Salimbeni, A. Techno-Economic Assessment of Lignocellulosic Biomass Energy Conversion by Slow Oxidative Pyrolysis. Master’s Thesis, University of Florence, Florence, Italy, 2016.
- Zhang, Y.; Cui, Y.; Chen, P.; Liu, S.; Zhou, N.; Ding, K.; Fan, L.; Peng, P.; Min, M.; Cheng, Y.; et al. Gasification Technologies and Their Energy Potentials; Elsevier B.V.: Amsterdam, The Netherlands, 2019.
- Dooley, S.; Won, S.H.; Dryer, F.L. Surrogate Fuels and Combustion Characteristics of Liquid Transportation Fuels; Elsevier B.V.: Amsterdam, The Netherlands, 2019.
- Westbrook, C.K. Chemical kinetics of hydrocarbon ignition in practical combustion systems. Proc. Combust. Inst. 2000, 28, 1563–1577.
- Caretto, L. Introduction to Combustion Today′s Class; Spring: Long Beach, CA, USA, 2010; pp. 1–22.
- Kim, D.; Yoshikawa, K.; Lee, K.; Park, K.Y. Investigation of the combustion characteristics of municipal solid wastes and their hydrothermally treated products via thermogravimetric analysis. J. Mater. Cycles Waste Manag. 2015, 17, 258–265.
- Ribeiro, J.M.C.; Godina, R.; Matias, J.C.D.O.; Nunes, L.J.R. Future perspectives of biomass torrefaction: Review of the current state-of-the-art and research development. Sustainability 2018, 10, 2323.
- Bergman, P.C.A.; Boersma, A.R.; Zwart, R.W.R.; Kiel, J.H. Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations (BIOCOAL); C-05-013; ECN: Petten, The Netherlands, 2005; pp. 1–72.
- Bach, Q.V.; Skreiberg, O. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677.
- Yue, Y.; Singh, H.; Singh, B.; Mani, S. Torrefaction of sorghum biomass to improve fuel properties. Bioresour. Technol. 2017, 232, 372–379.
- Tumuluru, J.S.; Sokhansanj, S.; Wright, C.T.; Kremer, T. GC Analysis of Volatiles and Other Products from Biomass Torrefaction Process; IntechOpen: London, UK, 2012.
- Basu, P. Chp. 04: Torrefaction; Biomass Gasification, Pyrolysis and Torrefaction: Halifax, NS, Canada, 2013; pp. 93–154. ISBN 9780128129920.
- White, R.H.; Dietenberger, M.A. Wood Products: Thermal Degradation and Fire. Encycl. Mater. Sci. Technol. 2001, 9712–9716.
- Acharya, B.; Pradhan, R.R.; Dutta, A. Qualitative and kinetic analysis of torrefaction of lignocellulosic biomass using DSC-TGA-FTIR. AIMS Energy 2015, 3, 760–773.
- Karki, S.; Poudel, J.; Oh, S.C. Thermal pre-treatment of sewage sludge in a lab-scale fluidized bed for enhancing its solid fuel properties. Appl. Sci. 2018, 8, 183.
- Minowa, T.; Kondo, T.; Sudirjo, S.T. Thermochemical liquefaction of Indonesian biomass residues. Biomass Bioenergy 1998, 14, 517–524.
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624.
- Zhou, Y.; Hu, C. Catalytic thermochemical conversion of algae and upgrading of algal oil for the production of high-grade liquid fuel: A review. Catalysts 2020, 10, 145.
- Lv, S.H. High-performance superplasticizer based on chitosan. Biopolym. Biotech. Admix. Eco Effic. Constr. Mater. 2016, 131–150.
- Gartner, E.M.; MacPhee, D.E. A physico-chemical basis for novel cementitious binders. Cem. Concr. Res. 2011, 41, 736–749.
- Weiner, B.; Baskyr, I.; Poerschmann, J.; Kopinke, F.D. Potential of the hydrothermal carbonization process for the degradation of organic pollutants. Chemosphere 2013, 92, 674–680.
- Yoganandham, S.T.; Sathyamoorthy, G.; Renuka, R.R. Emerging Extraction Techniques: Hydrothermal Processing; Elsevier Inc.: Amsterdam, The Netherlands, 2020.
- Ischia, G.; Fiori, L. Hydrothermal Carbonization of Organic Waste and Biomass: A Review on Process, Reactor, and Plant Modeling. Waste Biomass Valorization 2021, 12, 2797–2824.
- Tumuluru, J.S.; Sokhansanj, S.; Wright, C.T.; Kremer, T. GC Analysis of Volatiles and Other Products from Biomass Torrefaction Process; IntechOpen: London, UK, 2012.
- Basu, P. Chp. 04: Torrefaction; Biomass Gasification, Pyrolysis and Torrefaction: Halifax, NS, Canada, 2013; pp. 93–154. ISBN 9780128129920.
- White, R.H.; Dietenberger, M.A. Wood Products: Thermal Degradation and Fire. Encycl. Mater. Sci. Technol. 2001, 9712–9716.
- Acharya, B.; Pradhan, R.R.; Dutta, A. Qualitative and kinetic analysis of torrefaction of lignocellulosic biomass using DSC-TGA-FTIR. AIMS Energy 2015, 3, 760–773.
- Karki, S.; Poudel, J.; Oh, S.C. Thermal pre-treatment of sewage sludge in a lab-scale fluidized bed for enhancing its solid fuel properties. Appl. Sci. 2018, 8, 183.
- Minowa, T.; Kondo, T.; Sudirjo, S.T. Thermochemical liquefaction of Indonesian biomass residues. Biomass Bioenergy 1998, 14, 517–524.
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624.
- Zhou, Y.; Hu, C. Catalytic thermochemical conversion of algae and upgrading of algal oil for the production of high-grade liquid fuel: A review. Catalysts 2020, 10, 145.
- Lv, S.H. High-performance superplasticizer based on chitosan. Biopolym. Biotech. Admix. Eco Effic. Constr. Mater. 2016, 131–150.
- Gartner, E.M.; MacPhee, D.E. A physico-chemical basis for novel cementitious binders. Cem. Concr. Res. 2011, 41, 736–749.
- Weiner, B.; Baskyr, I.; Poerschmann, J.; Kopinke, F.D. Potential of the hydrothermal carbonization process for the degradation of organic pollutants. Chemosphere 2013, 92, 674–680.
- Yoganandham, S.T.; Sathyamoorthy, G.; Renuka, R.R. Emerging Extraction Techniques: Hydrothermal Processing; Elsevier Inc.: Amsterdam, The Netherlands, 2020.
- Ischia, G.; Fiori, L. Hydrothermal Carbonization of Organic Waste and Biomass: A Review on Process, Reactor, and Plant Modeling. Waste Biomass Valorization 2021, 12, 2797–2824.
