The practice of physical exercise (PE), especially strength training (ST), has health benefits in the healthy population; however, the literature is scarce in the recommendations related to the population with intellectual disability (ID). This study represents the first analysis on the topic and aims to examine the structure and efficacy of ST experimental intervention programs in individuals with ID. This systematic review was carried out between January and April 2021, using the PubMed, Web of Science, Scopus, and SPORTDiscus databases, according to the PRISMA guidelines.
- intellectual disabilities
- neuromuscular training
- physical exercise program
- resistance training
1. Introduction
ST aims to provoke adaptations in the skeletal musculature through overloads, providing an increase in the production of muscle strength and activity of glycolytic enzymes, as well as the production of adenosine triphosphate/phosphocreatine and adaptations in the nervous system, in order to increase the recruitment of motor units [1][2]. During ST, the lower and upper limbs move against a resistance provided by gravity, body weight, dumbbells, straps, weighted bars, or exercise machines [3][4][5]. This entire process results in cellular micro-lesions, mainly in the eccentric action phase, activating defense systems such as neutrophils, macrophages, and cytokines, which will generate reactive oxygen and nitrogen species [6]. These micro-sockets are important for the muscle recovery and regeneration process due to the fusion of satellite cells with a main cell, and the induction of protein synthesis metabolism and muscle tissue recovery [30]. Thereafter, ST seems to induce muscle skeletal adaptations as a result of overload, providing an increase in the production of muscular strength and other central nervous system adaptations [1][2].
2. Analysis on Results
2.1. Selection of Studies
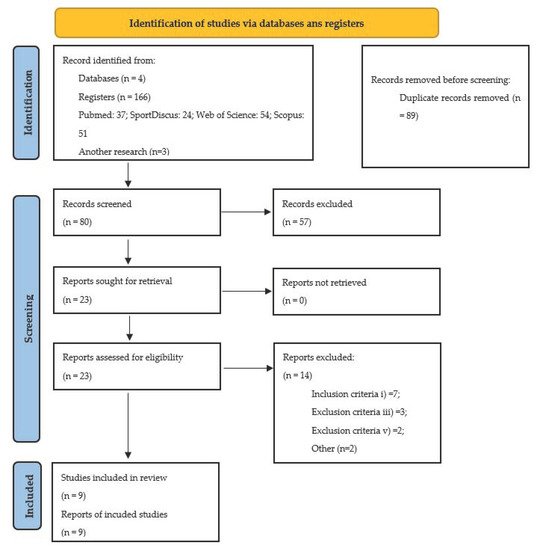
2.2. Characteristics of Studies
| Author (Year) | PEDro Scale | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Fornieles et al. [7] | s | 1 | 0 | 1 | - | - | 0 | 1 | 1 | 1 | 1 | 6 |
| Ghaeeni et al. [8] | s | 1 | 0 | 1 | - | - | 0 | 1 | 1 | 1 | 1 | 6 |
| Kachouri. et al. [9] | s | 1 | 0 | 1 | - | - | 0 | 1 | 1 | 1 | 1 | 6 |
| Neto et al. [10] | s | 1 | 0 | 1 | - | - | 0 | 1 | 1 | 1 | 1 | 6 |
| Ortiz-Ortiz et al. [11] | s | 1 | 0 | 1 | - | - | 0 | 1 | 1 | 1 | 1 | 6 |
| Rosety-Rodriguez. et al. [12] | s | 1 | 0 | 1 | - | - | 1 | 1 | 1 | 1 | 1 | 7 |
| Rosety-Rodriguez et al. [13] | s | 1 | 1 | 1 | - | - | 0 | 1 | 1 | 1 | 1 | 7 |
| Shields and Taylor [14] | s | 1 | 1 | 1 | - | - | 1 | 1 | 1 | 1 | 1 | 8 |
| Shields et al. [15] | s | 1 | 1 | 1 | - | - | 1 | 1 | 1 | 1 | 1 | 8 |
| Author, Year, Country | Aims | Participants | Study Design | Assessment Tools/Techniques |
|---|---|---|---|---|
| Fornieles et al. [7] Spain |
Influence of ST on salivary immunoglobulin A levels and hormone profile in sedentary DS adults. | N = 40 ♂; age: 23.7 ± 3.1; DS (IQ: 60–69); randomized groups: GE: N = 24 | CG: N = 16). |
Prospective cohort. | 8 RM test (exercises: bicep curl; leg extension; seated row; leg curl; triceps extension; leg press); saliva samples—analysis of immunoglobulin, testosterone, and cortisol (ELISA kits); box stacking test (ACSM, 2013; Smail and Horvat, 2006). |
| Ghaeeni et al. [8] Iran |
Effect of 8 weeks core stability training on static balance of DS children. | N = 16; age: 9.7 ± 1.7 y; DS; randomized groups: GE: N = 8 | CG: N = 8. |
Prospective cohort. | Static balance—stork test (Rahmani and Shahrokhi, 2011). |
| Kachouri et al. [9] Tunisia |
Effect of a combined strength and proprioception training program on muscle strength and postural balance in children with ID. | N = 20 ♂; age: 11.5 ± 1; ID (IQ: 50–70); randomized groups: GE: N = 10 | CG: N = 10. |
Prospective cohort. | Maximum voluntary contraction—quadríceps (dynamometer or manual muscle testing—Bohannon, 2005; Brinkmann, Andres, Medoza, and Sanjak, 1997); centre of pressure—static stabilometric platform (PostureWin©, Techno Concept®, Cereste, France; 12-bits A/D conversion). |
| Neto et al. [10] Brazil |
Effects of ST on body composition. | N = 15 (♂ = 11; ♀ = 4); age: 22.1 ± 7.5; DS; randomized groups: GE: ♂ = 6; ♀ = 2 CG: ♂ = 5; ♀ = 2. |
Prospective cohort. | Body mass—electronic scale model Filizola (Indústria Filizola S/A, São Paulo, Brazil); percentage of fat—seven thoracic, axillary, tricipital, subscapular, abdominal, supra-iliac, and thigh skinfolds (adipometer); fat mass calculated using the formula: body mass × percentage of fat/100; lean mass calculated using the formula: body mass − fat mass. |
| Ortiz-Ortiz et al. [11] Mexico |
Effect of a physical fitness program on body composition and isometric strength in DS children. |
N = 22; age: 11.8 ± 1.9 y; DS; randomized groups: GE: N = 13 | CG: N = 9. |
Prospective cohort. | Body weight—Tanita® InnerScan (BC-533, Tanita Corporation of America, Inc., Clearbrook, IL, USA); BMI = weight ÷ (height2); percentage of fat—subcutaneous triceps and calf sites; isometric strength—manual dynamometer—(dominant hand) MSD, (model SH5001, Düsseldorf, Germany). |
| Rosety-Rodriguez et al. [12] Spain |
Effect of ST on low-grade systemic inflammation in DS adults. | N = 40 ♂; age: 23.7 ± 3.1; DS (IQ: 60–69); randomized groups: GE: N = 24 | CG: N = 16. |
Prospective cohort. | Blood samples—plasma levels of leptin, adiponectin, interleukin-6 and TNF-α (ELISA kits); C-reactive protein—nephelometry; fat-free mass percentage—bio impedance (Tanita TBF521, Tanita Corporation of America, Inc., Clearbrook, IL, USA); waist circumference—anthropometric tape; time up-and-go test (Rikli and Jones, 1999). |
| Rosety-Rodriguez et al. [13] Spain |
Effect of ST on antioxidant defence system in sedentary DS. | N = 36 ♂; age: 28.1 ± 3.3; DS (mild ID–IQ: 60–69); randomized groups: GE: N = 18 | CG: N = 18. |
Prospective cohort. | 8 RM test (exercises: arm curl, leg extension, leg curl, low stroke, triceps extension and leg press); blood samples—puncture of the antecubital vein; maximum force—manual dynamometer JAMAR (Bolingbrook, IL, USA); peak torque of flexion and extension of the of the knees—isokinetic dynamometer at 90°/s -Technogym-REV 9000 (Technogym Spa, Gambettola, Italy); total antioxidant status of plasma—spectrophotometrically, Hitachi 902 Autoanalyzer (Roche, Alameda, CA, USA) by commercial kits (Randox, Crumlin, UK); reduced glutathione level after reaction with DTNB [(5,5-dithio-bis (2-nitrobenzoic acid)]; superoxide dismutase activity—xanthine oxidase-cytochrome c method; glutathione reductase activity; plasma ascorbate and α-tocopherol—reverse phase high-performance liquid chromatography. |
| Shields and Taylor [14] Australia |
Effects of ST on the ability to produce muscle strength and physical fitness. | N = 23 (♂ = 17; ♀ = 6); age: 15.6 ± 1.6; DS (mild to moderate ID); random groups: GE: N = 11 | CG: N = 12. |
Prospective cohort. | 1 RM test (chest and leg press); timed Up and Go test (Rikli and Jones, 1999); down stairs test (Zaino et al., 2004); grocery shelving task (Hill et al., 2004). |
| Shields et al. [15] Australia |
Effects of ST in adolescents and young DS adults. | N = 68 (♂ = 38; ♀ = 30); age: 17.9 ± 2.6; DS (mild to moderate ID); random groups: GE: N = 34 | CG: N = 34. |
Prospective cohort. | Box stacking test (ACSM, 2013); weighted pail carry test (ACSM, 2013); 1 RM test (chest and leg press). |
2.3. Origin
2.4. Participants
2.5. Evaluation Protocols/Instruments/Techniques
| Author, Year | Program Duration, Frequency, Session Duration |
Exercise Protocol | Results |
|---|---|---|---|
| Fornieles et al. [7] | 12 weeks; 3 × week; session duration ND. |
Exercises: arm curl; leg extension; seated row; leg curl; triceps extension; leg press; intensity: 40 a 65% 8 RM; 2 sets; 6 to 10 rep; 90 sec rest. |
Increased concentration of salivary immunoglobulin (p = 0.0120), testosterone levels (p = 0.0088) and job performance (p = 0.0141). |
| Ghaeeni et al. [8] | 8 weeks; 3 × week; 45 a 60 min/session. |
Abdominal workout; 3 to 4 exercises per session; 3 to 6 sets; 10 to 20 rep. |
Improvement of static balance (p = 0.0001). |
| Kachouri et al. [9] Tunisia |
8 weeks; 3 × week; 45 a 60 min/session. |
All exercises were performed in two surfaces, firm and foam; exercises included air squat, squat jumps, straight sit ups, power sit up, flutter kicks, two-foot ankle hop, single-foot side-to side ankle hop, tuck jump with knees up, standing long jump, double leg hops, single leg hops, standing on one-foot, lateral jump with both feet, lateral jump with one foot, running up the stairs with one foot and running up the stairs with both feet. 3 to 5 sets; 15 to 20 rep. |
Improves postural balance. |
| Neto et al. [10] | 12 weeks; 3 × week; 60 min/session. |
Exercises: chest press, squat, shoulders, leg curl, one-sided stroke, heel lift, bicipital curl, tricipital French e abdominal crunch; 3 sets; 8 to 12 rep; 30 to 60 sec rest. |
Increased lean mass (p = 0.008) and reduced fat percentage (p = 0.036). |
| Ortiz-Ortiz et al. [11] | 16 weeks; 5 × week; 55 min/session. |
Circuit exercises using weight disks, rubber bands, dumbbells, medical balls and shin guards with weights—biceps curl, triceps extension, chest press, and handgrip with different degrees of tension. | Reduction in the BMI (p < 0.0001) and the skin fold of the twin (p = 0.008); increased isometric strength (p < 0.0001). |
| Rosety-Rodriguez et al. [12] | 12 weeks; 3 × week; session duration ND. |
Exercises included arm curl, leg extension, seated row, leg curl, triceps extension, and leg press; 40 to 65% of 8 RM; 2 sets; 6 to 10 rep. |
Plasma levels of leptin (p < 0.05), TNF-α (p < 0.05) and IL-6 (p < 0.05) and waist circumference decreased (p = 0.0416); increase in fat-free mass (p = 0.011); improved response to systemic inflammation. |
| Rosety-Rodriguez et al. [13] | 12 weeks; 3 × week; Session duration ND. |
Exercises included arm curl, leg extension, seated row, leg curl, triceps extension, and leg press; 40 to 50% of 8 RM; 2 sets; 8 to 10 rep; 90s rest. |
Improvement of the antioxidant defense system; reduction in markers of oxidative damage. |
| Shields and Taylor [14] | 10 weeks; 2 × week; session duration ND. |
Exercises: lat pull-down, seated chest press, seated row, seated leg press, knee extension, calf raise; 3 sets; 12 rep or until fatigue; 2 min rest between exercises. |
Improvement in muscle strength of the lower limbs (mean difference 36 kg, 95% CI 15 to 58). |
| Shields et al. [15] | 10 weeks; 2 × week; 60 min/session. |
Exercises: lat pull-down, seated chest press, seated row, seated leg press, knee extension, seated calf raise; 3 sets; 12 rep; 60 to 80% RM; 2 min rest between exercises. |
Improvement in muscle strength of the lower 1imbs (mean difference 25 kg, 95% CI 8 to 42) and upper limbs 1 (mean difference 7 kg, 95% CI 3 to 11). |
2.6. Characteristics of the Strength Training Protocols
3. Current Insights
3.1. Program Duration
3.2. Frequency
3.3. Session Duration
3.4. Sets
3.5. Repetitions
3.6. Intensity
3.7. Exercises
3.8. ST Programs Outcomes
This entry is adapted from the peer-reviewed paper 10.3390/sports9090125
References
- Nogueira, A.C.; Simão, R.; Carvalho, M.C.G.A.; Vale, R.G.S.; Dantas, P.M.S.; Dantas, E.H.M. Concentração de hidroxiprolina como marcador bioquímico do dano músculo esquético após treinamento de resistência de força. Rev. Bras. Ciência Mov. 2008, 15, 33–38.
- Pereira, A.; Izquierdo, M.; Silva, A.J.; Costa, A.M.; Bastos, E.; González-Badillo, J.J.; Marques, M.C. Effects of high-speed power training on functional capacity and muscle performance in older women. Exp. Gerontol. 2012, 47, 250–255.
- Schoenfeld, B.J.; Ogborn, D.; Krieger, J.W. Effects of resistance training frequency on measures of muscle hypertrophy: A systematic review and meta-analysis. Sports Med. 2016, 46, 1689–1697.
- Lopes, J.S.S.; Machado, A.F.; Micheletti, J.K.; de Almeida, A.C.; Cavina, A.P.; Pastre, C.M. Effects of training with elastic resistance versus conventional resistance on muscular strength: A systematic review and meta-analysis. SAGE Open Med. 2019, 7, 2050312119831116.
- Chaves, T.S.; de Campos Biazon, T.M.P.; Dos Santos, L.M.E.; Libardi, C.A. Effects of resistance training with controlled versus self-selected repetition duration on muscle mass and strength in untrained men. PeerJ 2020, 8, e8697.
- Bloomer, R.J.; Goldfarb, A.H. Anaerobic exercise and oxidative stress: A review. Can. J. Appl. Physiol. 2004, 29, 245–263.
- Fornieles, G.; Rosety, M.A.; Elosegui, S.; Rosety, J.M.; Alvero-Cruz, J.R.; Garcia, N.; Rosety, M.; Rodriguez-Pareja, T.; Toro, R.; Rosety-Rodriguez, M.; et al. Salivary testosterone and immunoglobulin A were increased by resistance training in adults with Down syndrome. Braz. J. Med. Biol. Res. 2014, 47, 345–348.
- Ghaeeni, S.; Bahari, Z.; Khazaei, A. Effect of core stability training on static balance of the children with down syndrome. Phys. Tearments J. 2015, 5, 49–54.
- Kachouri, H.; Borji, R.; Baccouch, R.; Laatar, R.; Rebai, H.; Sahli, S. The effect of a combined strength and proprioceptive training on muscle strength and postural balance in boys with intellectual disability: An exploratory study. Res. Dev. Disabil. 2016, 53, 367–376.
- Neto, J.; Pontes, L.; Filho, J. Body compostion alterations resulting from weight training in subjects with down syndrome. Rev. Bras. Med. Esporte 2010, 16, 9–12.
- Ortiz-Ortiz, M.; Terrazas-Ordorica, K.; Cano-Rodríguez, L.; Gómez Miranda, L.; Ozols-Rosales, A.; Moncada-Jiménez, J. Effect of an intensive physical conditioning program on body composition and isometric strength in children with Down syndrome. J. Phys. Educ. Sport 2019, 19, 897–902.
- Rosety-Rodriguez, M.; Camacho, A.; Rosety, I.; Fornieles, G.; Rosety, M.A.; Diaz, A.J.; Rosety, M.; Ordonez, F.J. Resistance circuit training reduced inflammatory cytokines in a cohort of male adults with Down syndrome. Med. Sci. Monit. 2013, 19, 949–953.
- Rosety-Rodriguez, M.; Bernardi, M.; Elosegui, S.; Rosety, I.; Diaz, A.J.; Rosety, M.A.; Brenes, F.; Oliva-Pascual-Vaca, A.; Alvero-Cruz, J.R.; Ordonez, F.J. A short-term resistance training circuit improved antioxidants in sedentary adults with Down Syndrome. Oxidative Med. Cell. Longev. 2021, 2021, e8811153.
- Shields, N.; Taylor, N.F. A student-led progressive resistance training program increases lower limb muscle strength in adolescents with Down syndrome: A randomised controlled trial. J. Physiother. 2010, 56, 187–193.
- Shields, N.; Taylor, N.F.; Wee, E.; Wollersheim, D.; O’Shea, S.D.; Fernhall, B. A community-based strength training programme increases muscle strength and physical activity in young people with Down syndrome: A randomised controlled trial. Res. Dev. Disabil. 2013, 34, 4385–4394.
- ACSM. American College of Sports Medicine-Guidelines for Exercise Testing and Prescription, 10th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2017.
- Cunha, P.M.; Nunes, J.P.; Tomeleri, C.M.; Nascimento, M.A.; Schoenfeld, B.J.; Antunes, M.; Gobbo, L.A.; Teixeira, D.; Cyrino, E.S. Resistance training performed with single and multiple sets induces similar improvements in muscular strength, muscle mass, muscle quality, and igf-1 in older women: A randomized controlled trial. J. Strength Cond. Res. 2020, 34, 1008–1016.
- Iversen, V.M.; Norum, M.; Schoenfeld, B.J.; Fimland, M.S. No Time to Lift? Designing Time-Efficient Training Programs for Strength and Hypertrophy: A Narrative Review. Sports Med. 2021, 1–17.
- Duplanty, A.; Vingren, J.; Keller, J. Exercise training recommendations: Working with individuals with intellectual disabilities. Strength Cond. J. 2014, 36, 29–31.
- Aharoni, H. Adapted physical activities for the intellectually challenged adolescent: Psychomotor characteristics and implications for programming and motor intervention. Int. J. Adolesc. Med. Health 2005, 17, 33–47.
- Ayaso-Maneiro, J.; Domínguez-Prado, D.M.; García-Soidan, J.L. Influence of weight loss therapy programs in body image self-perception in adults with intellectual disabilities. Int. J. Clin. Health Psychol. 2014, 14, 178–185.
- Chaushu, S.; Yefenof, E.; Becker, A.; Shapira, J.; Chaushu, G. A link between parotid salivary Ig level and recurrent respiratory infections in young Down’s syndrome patients. Oral Microbiol. Immunol. 2002, 17, 172–176.
- Lotan, M. Quality Physical Intervention Activity for Persons with Down Syndrome. Sci. World J. 2007, 7, 7–19.
- Shields, N.; Taylor, N.F.; Fernhall, B. A study protocol of a randomised controlled trial to investigate if a community based strength training programme improves work task performance in young adults with Down syndrome. BMC Pediatr. 2010, 10, 17.
- Zenebe, K.; Legesse, K.; Mandal, S.; Mahmud, M.; Aragaw, K. Effects of sixteen week of resistance exercises on some selected cognitive variables development in adolescents with intellectual disabilities. Turk. J. Kinesiol. 2020, 6, 26–31.
- Jeon, B.; Son, S. Effects of the health management importance awareness on occupational performance and basic fitness among intellectually disabled participated the muscle strengthening exercise. J. Card. Pulm. Rehabil. 2017, 1, 1–5.
- Raulino, A.G.D.; Brito, C.J.; Barros, J.F. Efeito do treinamento com pesos nas atividades da vida diária em deficientes intelectuais. Rev. Bras. Ciências Esporte 2014, 36, 13–25.
- Schalock, R.; Verdugo, M. Handbook on Quality of Life for Human Service Practitioners; American Association on Mental Retardation: Washington, DC, USA, 2002.
- Martins, A.D.; Oliveira, R.; Brito, J.P.; Costa, T.; Ramalho, F.; Pimenta, N.; Santos-Rocha, R. Phase angle cutoff value as a marker of the health status and functional capacity in breast cancer survivors. Physiol. Behav. 2021, 235, 113400.
- Sardinha, L.B. Physiology of exercise and phase angle: Another look at BIA. Eur. J. Clin. Nutr. 2018, 72, 1323–1327.
- Dairo, Y.M.; Collett, J.; Dawes, H.; Oskrochi, G.R. Physical activity levels in adults with intellectual disabilities: A systematic review. Prev. Med. Rep. 2016, 4, 209–219.
- St. John, L.; Borschneck, G.; Cairney, J. A systematic review and meta-analysis examining the effect of exercise on individuals with intellectual disability. Am. J. Intellect Dev. Disabil. 2020, 125, 274–286.
- Hawke, T.J.; Garry, D.J. Myogenic satellite cells: Physiology to molecular biology. J. Appl. Physiol. 2001, 91, 534–551.
- Ratamess, N.; Alvar, B.; Evetoch, T.; Housh, T.; Kibler, W.; Kraemer, W. Progression models in resistance training for healthy adults [ACSM position stand]. Med. Sci. Sports Exerc. 2009, 41, 687–708.
- Cowley, P.M.; Ploutz-Snyder, L.L.; Baynard, T.; Heffernan, K.S.; Jae, S.Y.; Hsu, S.; Lee, M.; Pitetti, K.H.; Reiman, M.P.; Fernhall, B. The effect of progressive resistance training on leg strength, aerobic capacity and functional tasks of daily living in persons with Down syndrome. Disabil. Rehabil. 2011, 33, 2229–2236.
- Hecksteden, A.; Kraushaar, J.; Scharhag-Rosenberger, F.; Theisen, D.; Senn, S.; Meyer, T. Individual response to exercise training—A statistical perspective. J. Appl. Physiol. 2015, 118, 1450–1459.
