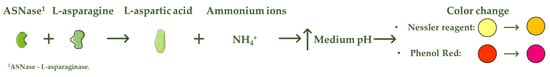
L-asparaginase (ASNase) is an aminohydrolase enzyme widely used in the pharmaceutical and food industries. Although currently its main applications are focused on the treatment of lymphoproliferative disorders such as acute lymphoblastic leukemia (ALL) and acrylamide reduction in starch-rich foods cooked at temperatures above 100 °C, its use as a biosensor in the detection and monitoring of L-asparagine levels is of high relevance. ASNase-based biosensors are a promising and innovative technology, mostly based on colorimetric detection since the mechanism of action of ASNase is the catalysis of the L-asparagine hydrolysis, which releases L-aspartic acid and ammonium ions, promoting a medium pH value change followed by color variation. ASNase biosensing systems prove their potential for L-asparagine monitoring in ALL patients, along with L-asparagine concentration analysis in foods, due to their simplicity and fast response.
- L-asparaginase
- biosensor
- L-asparagine
- monitoring
- ammonia
- pharmaceutical
- food
- industry
1. Introduction
2. Types and Applications of L-Asparaginase-Based Biosensors
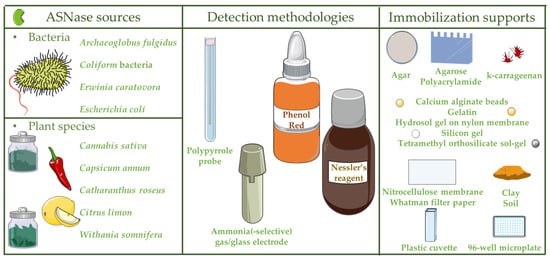
| Industry | Application | Detection Methodology | ASNase 1 Source | Immobilization Supports |
Ref. |
|---|---|---|---|---|---|
| __ | Ammonia sensing | Potentiometric ammonia gas detector | Escherichia coli | ― | [22] |
| __ | Ammonia sensing | Ammonia gas electrode | Garlic tissue cells | ― | [23] |
| __ | Ammonia sensing | Polypyrrole probe | E. coli | ― | [24] |
| Pharmaceutical | L-asparagine monitoring in ALL 2 patients |
Phenol red | Nitrocellulose membrane; silicone gel; calcium alginate beads | [25] | |
| Food | L-asparagine concentration analysis in foods |
Phenol red | Coliform bacterial cells |
Tetramethyl orthosilicate sol-gel | [26] |
| Ammonia sensing | Ammonium-selective glass electrode | Archaeoglobus fulgidus |
― | [27] | |
| Food; pharmaceutical | L-asparagine concentration analysis in foods; L-asparagine monitoring in ALL 2 patients |
Nessler’s reagent |
Erwinia carotovora |
Plastic cuvette | [28] |
| 96-well microplate | [29] | ||||
| Pharmaceutical | L-asparagine monitoring in ALL 2 patients |
Phenol red | Capsicum annum |
Gelatin; polyacrylamide; agar; calcium alginate beads | [30] |
| Citrus limon | Agar; agarose; gelatin; polyacrylamide; calcium alginate beads | [31] | |||
| Withania somnifera |
Gelatin; agarose; agar; calcium alginate beads | [21] | |||
| Cannabis sativa | Gelatin; agarose; agar; calcium alginate beads; Whatman filter paper; hydrosol gel on nylon membrane | [32] | |||
| Catharanthus roseus | Agar; soil; clay; k-carrageenan | [11] |
2.1. ASNase from Bacteria for the Development of Biosensors
2.1.1. Pharmaceutical Industry
2.1.2. Food Industry
2.2. ASNase from Plant Species for the Development of Biosensors
| Plant-ASNase 1-Based Biosensors | Immobilization Supports | L-Asparagine Detection Limit (M) | Response Time Range (s) | Response Time for Leukemic Blood Serum Samples (s) | Biocomponent Stability | Ref |
|---|---|---|---|---|---|---|
| C. annum-based biosensor | Gelatin | 10−9–10−1 | 10–21.6 | 20 | >15 days | [30] |
| Polyacrylamide | 10–20 | 18.7 | >1 month | |||
| Agar | 7.5–14.2 | 12.5 | >15 days | |||
| Calcium alginate beads | 7.1–12.3 | 11.2 | >4 months | |||
| C. limon-based biosensor | Agar | 10−10–10−1 | 6–14.2 | 13 | 1 month | [31] |
| Agarose | 9–16.4 | 16 | 25 days | |||
| Gelatin | 10–22 | 20 | 9 days | |||
| Polyacrylamide | 10–20 | 18 | 25 days | |||
| Calcium alginate beads | 7–12 | 11 | 3 months | |||
| W. somnifera-based biosensor | Gelatin | 10−10–10−1 | 10–22 | 19 ± 0.5 | >4 days | [21] |
| Agarose | 10–17 | 15 | >12 days | |||
| Agar | 7–14 | 12 | >4 days | |||
| Calcium alginate beads | 7–12 | 11 | >2 months | |||
| C. sativa-based biosensor | Gelatin | 10−10–10−1 | 8–21 | 19 | ― | [32] |
| Agarose | 9.17–16 | 15.8 | ― | |||
| Agar | 7.3–15 | 13.3 | ― | |||
| Calcium alginate beads | 7–11 | 11.1 | ― | |||
| Whatman filter paper | 11–23 | 21 | ― | |||
| Hydrosol gel on nylon membrane | 5–10 | 9 | >4 months | |||
| C. roseus-based biosensor | Agar | 10−10–10−1 | 7–14 | ― | ― | [11] |
| Soil | 4–12 | ― | ― | |||
| Clay | 3–11 | ― | ― | |||
| k-carrageenan | 3–10 | 7 | ― |
3. Conclusions and Future Perspectives
References
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification 1 International Union of Pure and Applied Chemistry: Physical Chemistry Division, Commission I.7 (Biophysical Chemistry); Analytical Chemistry Division, Commission V.5 (Electroanalytical). Biosens. Bioelectron. 2001, 16, 121–131.
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120.
- Turner, A.; Karube, I.; Wilson, G.S. Biosensors: Fundamentals and Applications, 1st ed.; Oxford University Press: Oxford, UK, 1987; ISBN 0198547242.
- Songa, E.A.; Okonkwo, J.O. Recent approaches to improving selectivity and sensitivity of enzyme-based biosensors for organophosphorus pesticides: A review. Talanta 2016, 155, 289–304.
- Zhilei, W.; Zaijun, L.; Xiulan, S.; Yinjun, F.; Junkang, L. Synergistic contributions of fullerene, ferrocene, chitosan and ionic liquid towards improved performance for a glucose sensor. Biosens. Bioelectron. 2010, 25, 1434–1438.
- Nunes, J.C.F.; Cristóvão, R.O.; Freire, M.G.; Santos-Ebinuma, V.C.; Faria, J.L.; Silva, C.G.; Tavares, A.P.M. Recent strategies and applications for L-asparaginase confinement. Molecules 2020, 25, 5827.
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of enzymes: A literature survey. In Ebolaviruses; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2013; Volume 1051, pp. 15–31. ISBN 9781627035491.
- Kidd, J.G. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. I. Course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J. Exp. Med. 1953, 98, 565–582.
- Kidd, J.G. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. II. Studies on the nature of the active serum constituent: Histological mechanism of the regression: Tests for effects of guinea pig serum on lymphoma cells in vitro. J. Exp. Med. 1953, 98, 583–606.
- Ando, M.; Sugimoto, K.; Kitoh, T.; Sasaki, M.; Mukai, K.; Ando, J.; Egashira, M.; Schuster, S.M.; Oshimi, K. Selective apoptosis of natural killer-cell tumours by L-asparaginase. Br. J. Haematol. 2005, 130, 860–868.
- Sandeep, P.; Raman, K.; Kuldeep, K. Enzyme based asparagine biosensor for the detection of asparagine levels in leukemic samples. Int. J. Appl. Biol. Pharm. Technol. 2015, 6, 40–43.
- Avramis, V.I.; Panosyan, E.H. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: The past, the present and recommendations for the future. Clin. Pharmacokinet. 2005, 44, 367–393.
- Paillassa, J.; Leguay, T.; Thomas, X.; Huguet, F.; Audrain, M.; Lheritier, V.; Vianey-Saban, C.; Acquaviva-Bourdain, C.; Pagan, C.; Dombret, H.; et al. Monitoring of asparagine depletion and anti-L-asparaginase antibodies in adult acute lymphoblastic leukemia treated in the pediatric-inspired GRAALL-2005 trial. Blood Cancer J. 2018, 8, 45.
- World Health Organization. IARC monographs on the evaluation of carcinogenic risks to humans—Volume 63. Dry cleaning, some chlorinated solvents and other industrial chemicals. Anal. Chim. Acta 1996, 336, 229–230.
- Joint Food and Agriculture Organization; World Health Organization. Health Implications of Acrylamide in Food: Report of a Joint FAO/WHO Consultation, WHO Headquarters, Geneva, Switzerland, 25–27 June 2002; World Health Organization: Geneva, Switzerland, 2002; ISBN 9241562188.
- Abt, E.; Robin, L.P.; McGrath, S.; Srinivasan, J.; DiNovi, M.; Adachi, Y.; Chirtel, S. Acrylamide levels and dietary exposure from foods in the United States, an update based on 2011–2015 data. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 1475–1490.
- Tardiff, R.G.; Gargas, M.L.; Kirman, C.R.; Leigh Carson, M.; Sweeney, L.M. Estimation of safe dietary intake levels of acrylamide for humans. Food Chem. Toxicol. 2010, 48, 658–667.
- Xu, F.; Oruna-Concha, M.-J.; Elmore, J.S. The use of asparaginase to reduce acrylamide levels in cooked food. Food Chem. 2016, 210, 163–171.
- Parker, J.K.; Balagiannis, D.P.; Higley, J.; Smith, G.; Wedzicha, B.L.; Mottram, D.S. Kinetic model for the formation of acrylamide during the finish-frying of commercial french fries. J. Agric. Food Chem. 2012, 60, 9321–9331.
- Zubavichus, Y.; Fuchs, O.; Weinhardt, L.; Heske, C.; Umbach, E.; Denlinger, J.D.; Grunze, M. Soft X-ray-induced decomposition of amino acids: An XPS, mass spectrometry, and NEXAFS study. Radiat. Res. 2004, 161, 346–358.
- Kumar, K.; Kataria, M.; Verma, N. Plant asparaginase-based asparagine biosensor for leukemia. Artif. Cells Nanomed. Biotechnol. 2013, 41, 184–188.
- Fraticelli, Y.M.; Meyerhoff, M.E. On-Line gas dialyzer for automated enzymatic analysis with potentiometric ammonia detection. Anal. Chem. 1983, 55, 359–364.
- Kim, S.J.; Kim, G.M.; Bae, Y.J.; Lee, E.Y.; Hur, M.H.; Ahn, M.K. Determination of L-asparagine using a garlic tissue electrode. Yakhak Hoeji 1995, 39, 113–117.
- Ren, J.; He, F.; Zhang, L. The construction and application of a new PPY-MSPQC for L-asparaginase activity assay. Sens. Actuators B Chem. 2010, 145, 272–277.
- Verma, N.; Kumar, K.; Kaur, G.; Anand, S. E. coli K-12 asparaginase-based asparagine biosensor for leukemia. Artif. Cells Blood Substit. Biotechnol. 2007, 35, 449–456.
- Verma, N.; Bansal, M.; Kumar, S. Whole cell based miniaturized fiber optic biosensor to monitor L-asparagine. Adv. Appl. Sci. Res. 2012, 3, 809–814.
- Li, J.; Wang, J.; Bachas, L.G. Biosensor for asparagine using a thermostable recombinant asparaginase from Archaeoglobus fulgidus. Anal. Chem. 2002, 74, 3336–3341.
- Kotzia, G.A.; Labrou, N.E. Engineering substrate specificity of E. carotovora L-asparaginase for the development of biosensor. J. Mol. Catal. B Enzym. 2011, 72, 95–101.
- Labrou, N.E.; Muharram, M.M. Biochemical characterization and immobilization of Erwinia carotovora L-asparaginase in a microplate for high-throughput biosensing of L-asparagine. Enzym. Microb. Technol. 2016, 92, 86–93.
- Kumar, K.; Walia, S. L-asparaginase extracted from Capsicum annum L and development of asparagine biosensor for leukemia. Sens. Transducers 2012, 144, 192–200.
- Kumar, K.; Punia, S.; Kaur, J.; Pathak, T. Development of plant asparagine biosensor for detection of leukemia. J. Pharm. Biomed. Sci. 2013, 35, 1796–1801.
- Teena, P.; Raman, K.; Jagjit, K.; Kuldeep, K. Isolation of L-asparaginase from Cannabis Sativa and development of biosensor for detection of asparagine in leukemic serum samples. Res. J. Pharm. Technol. 2014, 7, 850–855.
- Cachumba, J.J.M.; Antunes, F.A.F.; Peres, G.F.D.; Brumano, L.P.; Dos Santos, J.C.; Da Silva, S.S. Current applications and different approaches for microbial L-asparaginase production. Braz. J. Microbiol. 2016, 47, 77–85.
- Castro, D.; Marques, A.S.C.; Almeida, M.R.; de Paiva, G.B.; Bento, H.B.S.; Pedrolli, D.B.; Freire, M.G.; Tavares, A.P.M.; Santos-Ebinuma, V.C. L-asparaginase production review: Bioprocess design and biochemical characteristics. Appl. Microbiol. Biotechnol. 2021, 105, 4515–4534.
- Sokolov, N.N.; Eldarov, M.A.; Pokrovskaya, M.V.; Aleksandrova, S.S.; Abakumova, O.Y.; Podobed, O.V.; Melik-Nubarov, N.S.; Kudryashova, E.V.; Grishin, D.V.; Archakov, A.I. Bacterial recombinant L-asparaginases: Properties, structure, and anti-proliferative activity. Biochem. Moscow Suppl. Ser. B 2015, 9, 325–338.
- Xu, J.; Lee, H. Anti-biofouling strategies for long-term continuous use of implantable biosensors. Chemosensors 2020, 8, 66.
- Wisniewski, N.; Moussy, F.; Reichert, W.M. Characterization of implantable biosensor membrane biofouling. Fresenius J. Anal. Chem. 2000, 366, 611–621.
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme biosensors for biomedical applications: Strategies for safeguarding analytical performances in biological fluids. Sensors 2016, 16, 780.
- Lin, P.-H.; Li, B.-R. Antifouling strategies in advanced electrochemical sensors and biosensors. Analyst 2020, 145, 1110–1120.






