Trefoil factor family (TFF) peptides mainly consist of characteristic TFF domains, which contain about 40 amino acid residues, including 6 conserved cysteine residues. TFF peptides possess a single (mammalian TFF1 and TFF3), two (mammalian TFF2, Xenopus laevis xP2) or four TFF domains (X. laevis xP4). They exhibit lectin activities and are characteristic exocrine products of the mucous epithelia. Here, they play different roles for mucosal protection and the innate immune defense: TFF1 is a gastric tumor suppressor; TFF2 builds a lectin complex with the mucin MUC6, physically stabilizing the inner gastric mucus layer; and TFF3 forms a disulfide-linked heterodimer with IgG Fc binding protein (FCGBP), probably preventing the infiltration of microorganisms. Minor amounts of TFF peptides are endocrine products of the immune and nervous systems. Pathologically, TFF peptides are linked to inflammation. There are increasing indications that TFF peptides can antagonize cytokine receptors, such as receptors for IL-1β, IL-6, and TNFα (thereby acting as anti-inflammatory peptides). TFF peptides can probably also activate a variety of receptors, such as CXCR4. The TFF domain is a unique shuffled module which is also present in a number of mosaic proteins, such as zona pellucida proteins, sugar degrading enzymes and frog skin mucins. Here, their function seems to be defined by a lectin activity, which might even allow a role in fertilization.
- TFF-domain
- lectin
- inflammation
- cytokine receptors
- innate immune defense
- gastric cancer
- re-active oxygen species
- macrophages
1. Introduction or History
2. Structure and Expression
2.1. Genomic Organization, Structure and Natural Forms of TFF Peptides
2.2. Mosaic Proteins Containing TFF Domains
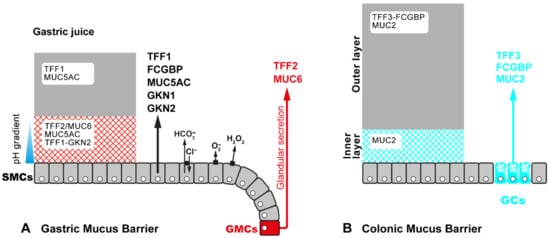
2.3. Exocrine Secretion of TFF Peptides in Mucous Epithelia
2.4. Endocrine Secretion of TFF Peptides
2.5. Pathological Expression of TFF Peptides: Links to Inflammation and Cancer
3. Functional Aspects
3.1. TFF Domains Have Different Lectin Activities
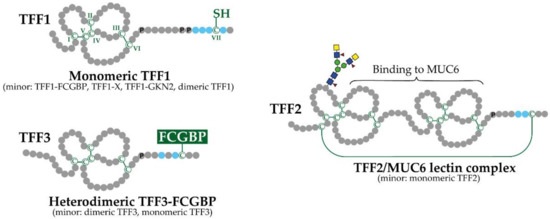
3.2. Exocrine TFF Peptides Occur in Different Molecular Forms and Have Diverse Molecular Functions
3.3. Tff-Deficient (TffKO) Mice Have Different Phenotypes: Functional Implications
3.3.1. TFF1 Is a Gastric Tumor Suppressor
3.3.2. TFF2: Component of the Gastric Mucus Barrier, Anti-Inflammatory Peptide, and Induction of IL-33 (Promotion of Th2 Immunity)
3.3.3. TFF3: Component of the Intestinal Mucus Barrier
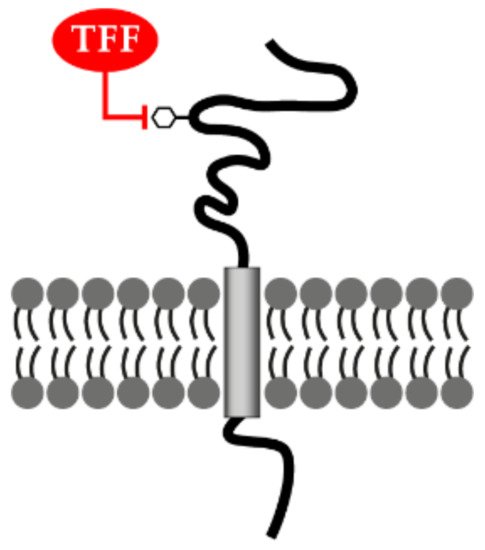
3.4. TFF Peptides Weakly Enhance Cell Migration: TFF Binding Sites and Hypothetical Lectin-Triggered Activation of Transmembrane Glycoproteins
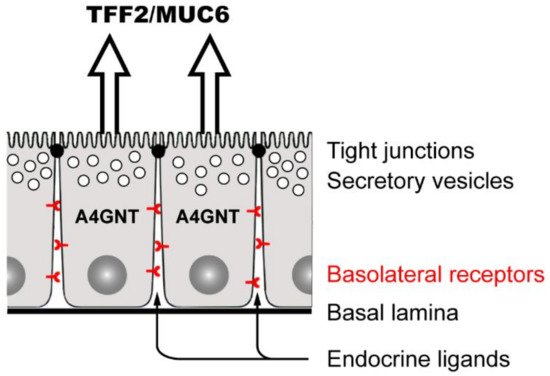
3.5. Lectin-Triggered Receptor Blocking Hypothesis (Anti-Inflammatory Action)
4. Medical Perspectives
References
- Thim, L. A new family of growth factor-like peptides. ‘Trefoil’ disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins). FEBS Lett. 1989, 250, 85–90.
- Poulsom, R.; Wright, N.A. Trefoil peptides: A newly recognized family of epithelial mucin-associated molecules. Am. J. Physiol. Liver Physiol. 1993, 265, G205–G213.
- Hoffmann, W.; Hauser, F. The P-domain or trefoil motif: A role in renewal and pathology of mucous epithelia? Trends Biochem. Sci. 1993, 18, 239–243.
- Sands, B.E.; Podolsky, D.K. The trefoil peptide family. Annu. Rev. Physiol. 1996, 58, 253–273.
- Thim, L. Trefoil peptides: From structure to function. Cell. Mol. Life Sci. 1997, 53, 888.
- Ribieras, S.; Tomasetto, C.; Rio, M.C. The pS2/TFF1 trefoil factor, from basic research to clinical applications. Biochim. Biophys. Acta 1998, 1378, F61–F77.
- Wong, W.M.; Poulsom, R.; Wright, N.A. Trefoil peptides. Gut 1999, 44, 890–895.
- Taupin, D.; Podolsky, D.K. Trefoil factors: Initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 2003, 4, 721–732.
- Thim, L.; May, F.E.B. Structure of mammalian trefoil factors and functional insights. Cell. Mol. Life Sci. 2005, 62, 2956–2973.
- Kjellev, S. The trefoil factor family—Small peptides with multiple functionalities. Cell. Mol. Life Sci. 2008, 66, 1350–1369.
- Aihara, E.; Engevik, K.A.; Montrose, M.H. Trefoil factor peptides and gastrointestinal function. Annu. Rev. Physiol. 2017, 79, 357–380.
- Hoffmann, W. Trefoil factor family (TFF) peptides and their diverse molecular functions in mucus barrier protection and more: Changing the paradigm. Int. J. Mol. Sci. 2020, 21, 4535.
- Hoffmann, W. Trefoil factor family (TFF) peptides and their links to inflammation: A re-evaluation and new medical perspectives. Int. J. Mol. Sci. 2021, 22, 4909.
- Hoffmann, W. Trefoil factor family (TFF) peptides and their different roles in the mucosal innate immune defense and more: An update. Curr. Med. Chem. 2021, 28.
- Masiakowski, P.; Breathnach, R.; Bloch, J.; Gannon, F.; Krust, A.; Chambon, P. Cloning of cDNA sequences of hor-mone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982, 10, 7895–7903.
- Jørgensen, K.H.; Thim, L.; Jacobsen, H.E. Pancreatic spasmolytic polypeptide (PSP): I. Preparation and initial chemical char-acterization of a new polypeptide from porcine pancreas. Regul. Pept. 1982, 3, 207–219.
- Hoffmann, W. A new repetitive protein from Xenopus laevis skin highly homologous to pancreatic spasmolytic polypeptide. J. Biol. Chem. 1988, 263, 7686–7690.
- Suemori, S.; Lynch-Devaney, K.; Podolsky, D.K. Identification and characterization of rat intestinal trefoil factor: Tissue and cell-specific member of the trefoil protein family. Proc. Natl. Acad. Sci. USA 1991, 88, 11017–11021.
- Hauser, F.; Poulsom, R.; Chinery, R.; Rogers, L.A.; Hanby, A.M.; Wright, N.; Hoffmann, W. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc. Natl. Acad. Sci. USA 1993, 90, 6961–6965.
- Podolsky, D.; Lynch-Devaney, K.; Stow, J.; Oates, P.; Murgue, B.; DeBeaumont, M.; Sands, B.; Mahida, Y. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J. Biol. Chem. 1993, 268, 6694–6702.
- Hoffmann, W.; Jagla, W. Cell type specific expression of secretory TFF peptides: Colocalization with mucins and synthesis in the brain. Int. Rev. Cytol. 2002, 213, 147–181, 182e–188e.
- Wright, N.; Hoffmann, W.; Otto, W.; Rio, M.-C.; Thim, L. Rolling in the clover: Trefoil factor family (TFF)-domain peptides, cell migration and cancer. FEBS Lett. 1997, 408, 121–123.
- Sommer, P.; Blin, N.; Gött, P. Tracing the evolutionary origin of the TFF-domain, an ancient motif at mucous surfaces. Gene 1999, 236, 133–136.
- Hoffmann, W. TFF Peptides. In Handbook of Biologically Active Peptides, 2nd ed.; Karstin, A.J., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 1338–1345.
- Heuer, F.; Stürmer, R.; Heuer, J.; Kalinski, T.; Lemke, A.; Meyer, F.; Hoffmann, W. Different Forms of TFF2, A Lectin of the Human Gastric Mucus Barrier: In vitro binding studies. Int. J. Mol. Sci. 2019, 20, 5871.
- Znalesniak, E.B.; Salm, F.; Hoffmann, W. Molecular alterations in the stomach of Tff1-deficient mice: Early steps in antral carcinogenesis. Int. J. Mol. Sci. 2020, 21, 644.
- Hanisch, F.-G.; Ragge, H.; Kalinski, T.; Meyer, F.; Kalbacher, H.; Hoffmann, W. Human gastric TFF2 peptide contains an N-linked fucosylated N,N’-diacetyllactosediamine (LacdiNAc) oligosaccharide. Glycobiology 2012, 23, 2–11.
- Stürmer, R.; Reising, J.; Hoffmann, W. The TFF peptides xP1 and xP4 appear in distinctive forms in the Xenopus laevis gastric mucosa: Indications for different protective functions. Int. J. Mol. Sci. 2019, 20, 6052.
- Heuer, J.; Heuer, F.; Stürmer, R.; Harder, S.; Schlüter, H.; Emidio, N.B.; Muttenthaler, M.; Jechorek, D.; Meyer, F.; Hoffmann, W. The tumor suppressor TFF1 occurs in different forms and interacts with multiple partners in the human gastric mucus barrier: Indications for diverse protective functions. Int. J. Mol. Sci. 2020, 21, 2508.
- Westley, B.R.; Griffin, S.M.; May, F.E.B. Interaction between TFF1, a gastric tumor suppressor trefoil protein, and TFIZ1, a brichos domain-containing protein with homology to SP-C. Biochemistry 2005, 44, 7967–7975.
- Kouznetsova, I.; Laubinger, W.; Kalbacher, H.; Kalinski, T.; Meyer, F.; Roessner, A.; Hoffmann, W. Biosynthesis of gastrokine-2 in the human gastric mucosa: Restricted spatial expression along the antral gland axis and differential interaction with TFF1, TFF2 and mucins. Cell. Physiol. Biochem. 2007, 20, 899–908.
- Stürmer, R.; Müller, S.; Hanisch, F.-G.; Hoffmann, W. Porcine gastric TFF2 is a mucus constituent and differs from pancreatic TFF2. Cell. Physiol. Biochem. 2014, 33, 895–904.
- Stürmer, R.; Harder, S.; Schlüter, H.; Hoffmann, W. Commercial porcine gastric mucin preparations, also used as artificial saliva, are a rich source for the lectin TFF2: In vitro binding studies. ChemBioChem 2018, 19, 2598–2608.
- Hoffmann, W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more (Review). Int. J. Oncol. 2015, 47, 806–816.
- Albert, T.K.; Laubinger, W.; Müller, S.; Hanisch, F.-G.; Kalinski, T.; Meyer, F.; Hoffmann, W. Human Intestinal TFF3 Forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J. Proteome Res. 2010, 9, 3108–3117.
- Houben, T.; Harder, S.; Schlüter, H.; Kalbacher, H.; Hoffmann, W. Different forms of TFF3 in the human saliva: Heterodi-merization with IgG Fc binding protein (FCGBP). Int. J. Mol. Sci. 2019, 20, 5000.
- Hauser, F.; Hoffmann, W. xP1 and xPP-domain peptides expressed in Xenopus laevis stomach mucosa. J. Biol. Chem. 1991, 266, 21306–21309.
- Jagla, W.; Wiede, A.; Hoffmann, W.; Kölle, S. Differential expression of the TFF-peptides xP1 and xP4 in the gastrointestinal tract of Xenopus laevis. Cell Tissue Res. 1997, 291, 13–18.
- Botzler, C.; Oertel, M.; Hinz, M.; Hoffmann, W. Structure of the Xenopus laevis TFF-gene xP4.1, differentially expressed to its duplicated homolog xP4. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1999, 1489, 345–353.
- Hoffmann, W.; Jagla, W.; Wiede, A. Molecular medicine of TFF-peptides: From gut to brain. Histol. Histopathol. 2001, 16, 319–334.
- Madsen, J.; Nielsen, O.; Tornøe, I.; Thim, L.; Holmskov, U. Tissue localization of human trefoil factors 1, 2, and 3. J. Histochem. Cytochem. 2007, 55, 505–513.
- Cook, G.; Familari, M.; Thim, L.; Giraud, A. The trefoil peptides TFF2 and TFF3 are expressed in rat lymphoid tissues and participate in the immune response. FEBS Lett. 1999, 456, 155–159.
- Baus-Loncar, M.; Kayademir, T.; Takaishi, S.; Wang, T. Trefoil factor family 2 deficiency and immune response. Cell. Mol. Life Sci. 2005, 62, 2947–2955.
- Kurt-Jones, E.A.; Cao, L.; Sandor, F.; Rogers, A.B.; Whary, M.T.; Nambiar, P.R.; Cerny, A.; Bowen, G.; Yan, J.; Takaishi, S.; et al. Trefoil family factor 2 is expressed in murine gastric and immune cells and controls both gastrointestinal inflammation and systemic immune responses. Infect. Immun. 2007, 75, 471–480.
- Probst, J.C.; Skutella, T.; Müller-Schmid, A.; Jirikowski, G.F.; Hoffmann, W. Molecular and cellular analysis of rP1.B in the rat hypothalamus: In situ hybridization and immunohistochemistry of a new P-domain neuropeptide. Mol. Brain Res. 1995, 33, 269–276.
- Wright, N.A. Trefoil peptides and the gut. Gut 1993, 34, 577–579.
- Wong, W.M.; Playford, R.J.; Wright, N.A. Peptide gene expression in gastrointestinal mucosal ulceration: Ordered sequence or redundancy? Gut 2000, 46, 286–292.
- Hoffmann, W. TFF (trefoil factor family) peptides and their potential roles for differentiation processes during airway remod-eling. Curr. Med. Chem. 2007, 14, 2716–2719.
- Goldenring, J.R.; Nam, K.T.; Wang, T.C.; Mills, J.C.; Wright, N. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: Time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 2010, 138, 2207–2210.e1.
- May, F.E.; Westley, B.R. Trefoil proteins: Their role in normal and malignant cells. J. Pathol. 1997, 183, 4–7.
- Katoh, M. Trefoil factors and human gastric cancer (Review). Int. J. Mol. Med. 2003, 12, 3–9.
- Emami, S.; Rodrigues, S.; Rodrigue, C.M.; Le Floch, N.; Rivat, C.; Attoub, S.; Bruyneel, E.; Gespach, C. Trefoil factor family (TFF) peptides and cancer progression. Peptides 2004, 25, 885–898.
- Regalo, G.; Wright, N.A.; Machado, J.C. Trefoil factors: From ulceration to neoplasia. Cell. Mol. Life Sci. 2005, 62, 2910–2915.
- Perry, J.K.; Kannan, N.; Grandison, P.M.; Mitchell, M.D.; Lobie, P.E. Are trefoil factors oncogenic? Trends Endocrinol. Metab. 2008, 19, 74–81.
- Beck, S.; Sommer, P.; Blin, N.; Gött, P. 5′-flanking motifs control cell-specific expression of trefoil factor genes (TFF). Int. J. Mol. Med. 1998, 2, 353–414.
- Baus-Loncar, M.; Giraud, A.S. Multiple regulatory pathways for trefoil factor (TFF) genes. Cell. Mol. Life Sci. 2005, 62, 2921–2931.
- Giraud, A.S.; Jackson, C.; Menheniott, T.R.; Judd, L.M. Differentiation of the gastric mucosa IV. Role of trefoil peptides and IL-6 cytokine family signaling in gastric homeostasis. Am. J. Physiol. Liver Physiol. 2007, 292, G1–G5.
- Järvå, M.A.; Lingford, J.; John, A.; Soler, N.M.; Scott, N.E.; Goddard-Borger, E.D. Trefoil factors share a lectin activity that defines their role in mucus. Nat. Commun. 2020, 11, 2265.
- Reeves, E.P.; Ali, T.; Leonard, P.; Hearty, S.; O’Kennedy, R.; May, F.E.B.; Westley, B.R.; Josenhans, C.; Rust, M.; Suerbaum, S.; et al. Helicobacter pylori lipopolysaccharide interacts with TFF1 in a pH-dependent manner. Gastroenterology 2008, 135, 2043–2054.
- Clyne, M.; May, F.E.B. The interaction of helicobacter pylori with TFF1 and its role in mediating the tropism of the bacteria within the stomach. Int. J. Mol. Sci. 2019, 20, 4400.
- Emidio, N.B.; Baik, H.; Lee, D.; Stuermer, R.; Heuer, J.; Elliott, A.G.; Blaskovich, M.A.T.; Haupenthal, K.; Tegtmeyer, N.; Hoffmann, W.; et al. Chemical synthesis of human trefoil factor 1 (TFF1) and its homodimer provides novel insights into their mechanisms of action. Chem. Commun. 2020, 56, 6420–6423.
- Hanisch, F.-G.; Bonar, D.; Schloerer, N.; Schroten, H. Human trefoil factor 2 is a lectin that binds α-GlcNAc-capped mucin glycans with antibiotic activity against Helicobacter pylori. Cell. Physiol. Biochem. 2014, 289, 27363–27375.
- Stürmer, R.; Reising, J.; Hoffmann, W. Trefoil factor family (TFF) modules are characteristic constituents of separate mucin complexes in the xenopus laevis integumentary mucus: In vitro binding studies with FIM-A. 1. Int. J. Mol. Sci. 2020, 21, 2400.
- Schwarz, H.; Hoffmann, W. Subcellular localization of the TFF peptides xP1 and xP4 in the Xenopus laevis gastric/esophageal mucosa: Different secretion modes reflecting diverse protective functions. Int. J. Mol. Sci. 2020, 21, 761.
- Thim, L.; Madsen, F.; Poulsen, S.S. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur. J. Clin. Investig. 2002, 32, 519–527.
- Lang, T.; Klasson, S.; Larsson, E.; Johansson, M.E.; Hansson, G.C.; Samuelsson, T. Searching the evolutionary origin of epi-thelial mucus protein components-mucins and FCGBP. Mol. Biol. Evol. 2016, 33, 1921–1936.
- Lencer, W.I.; Blumberg, R.S. A passionate kiss, then run: Exocytosis and recycling of IgG by FcRn. Trends Cell Biol. 2005, 15, 5–9.
- Schwartz, J.L. Fcgbp—A potential viral trap in RV144. Open AIDS J. 2014, 8, 21–24.
- Madsen, J.; Sørensen, G.L.; Nielsen, O.S.; Tornøe, I.; Thim, L.; Fenger, C.; Mollenhauer, J.; Holmskov, U. A variant form of the human deleted in malignant brain tumor 1 (DMBT1) gene shows increased expression in inflammatory bowel diseases and interacts with dimeric trefoil factor 3 (TFF3). PLoS ONE 2013, 8, e64441.
- Madsen, J.; Mollenhauer, J.; Holmskov, U. Review: Gp-340/DMBT1 in mucosal innate immunity. Innate Immun. 2010, 16, 160–167.
- Tomasetto, C.; Rio, M.C. Pleiotropic effects of Trefoil Factor 1 deficiency. Cell. Mol. Life Sci. 2005, 62, 2916–2920.
- Lefebvre, O.; Chenard, M.-P.; Masson, R.; Linares, J.; Dierich, A.; LeMeur, M.; Wendling, C.; Tomasetto, C.; Chambon, P.; Rio, M.-C. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science 1996, 274, 259–262.
- Soutto, M.; Belkhiri, A.; Piazuelo, M.B.; Schneider, B.G.; Peng, D.; Jiang, A.; Washington, M.K.; Kokoye, Y.; Crowe, S.E.; Zaika, A.; et al. Loss of TFF1 is associated with activation of NF-kappaB-mediated inflammation and gastric neoplasia in mice and humans. J. Clin. Investig. 2011, 121, 1753–1767.
- Saukkonen, K.; Tomasetto, C.; Narko, K.; Rio, M.-C.; Ristimäki, A. Cyclooxygenase-2 expression and effect of celecoxib in gastric adenomas of trefoil factor 1-deficient mice. Cancer Res. 2003, 63, 3032–3036.
- Thiem, S.; Eissmann, M.F.; Elzer, J.; Jonas, A.; Putoczki, T.L.; Poh, A.; Nguyen, P.; Preaudet, A.; Flanagan, D.; Vincan, E.; et al. Stomach-specific activation of oncogenic KRAS and STAT3-dependent in-flammation cooperatively promote gastric tumorigenesis in a preclinical model. Cancer Res. 2016, 76, 2277–2287.
- Torres, L.-F.; Karam, S.M.; Wendling, C.; Chenard, M.-P.; Kershenobich, D.; Tomasetto, C.; Rio, M.-C. TreFoil factor 1 (TFF1/pS2) deficiency activates the unfolded protein response. Mol. Med. 2002, 8, 273–282.
- Farrell, J.J.; Taupin, D.; Koh, T.J.; Chen, D.; Zhao, C.M.; Podolsky, D.K.; Wang, T.C. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J. Clin. Investig. 2002, 109, 193–204.
- Fox, J.G.; Rogers, A.B.; Whary, M.T.; Ge, Z.; Ohtani, M.; Jones, E.K.; Wang, T.C. Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2-/-C57BL6 x Sv129 Helicobacter pylori-infected mice. Am. J. Pathol. 2007, 171, 1520–1528.
- McBerry, C.; Egan, C.E.; Rani, R.; Yang, Y.; Wu, D.; Boespflug, N.; Boon, L.; Butcher, B.; Mirpuri, J.; Hogan, S.P.; et al. Trefoil Factor 2 negatively regulates Type 1 immunity against toxoplasma gondii. J. Immunol. 2012, 189, 3078–3084.
- Wills-Karp, M.; Rani, R.; Dienger, K.; Lewkowich, I.; Fox, J.G.; Perkins, C.; Lewis, L.; Finkelman, F.D.; Smith, D.E.; Bryce, P.J.; et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J. Exp. Med. 2012, 209, 607–622.
- Buzzelli, J.N.; Chalinor, H.V.; Pavlic, D.I.; Sutton, P.; Menheniott, T.R.; Giraud, A.S.; Judd, L.M. IL33 is a stomach alarmin that initiates a skewed Th2 response to injury and infection. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 203–221.e3.
- Mashimo, H.; Wu, D.-C.; Podolsky, D.K.; Fishman, M.C. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 1996, 274, 262–265.
- Beck, P.L.; Wong, J.F.; Li, Y.; Swaminathan, S.; Xavier, R.J.; Devaney, K.L.; Podolsky, D.K. Chemotherapy-and radiothera-py-induced intestinal damage is regulated by intestinal trefoil factor. Gastroenterology 2004, 126, 796–808.
- Johansson, M.E.V.; Gustafsson, J.K.; Sjöberg, K.E.; Petersson, J.; Holm, L.; Sjövall, H.; Hansson, G.C. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE 2010, 5, e12238.
- Hoffmann, W. TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell. Mol. Life Sci. 2005, 62, 2932–2938.
- Cho, S.Y.; Klemke, R.L. Extracellular-regulated kinase activation and Cas/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J. Cell Biol. 2000, 149, 223–236.
- Otto, W.R.; Thim, L. Trefoil factor family-interacting proteins. Cell. Mol. Life Sci. 2005, 62, 2939–2946.
- Tajadura-Ortega, V.; Gambardella, G.; Skinner, A.; Halim, A.; Coillie, J.V.; Schjoldager, K.T.-B.G.; Beatson, R.; Graham, R.; Achkova, D.; Taylor-Papadimitriou, J.; et al. O-linked mucin-type glycosylation regulates the tran-scriptional programme downstream of EGFR. Glycobiology 2021, 31, 200–210.
- Poulsen, S.S.; Thulesen, J.; Nexø, E.; Thim, L. Distribution and metabolism of intravenously administered trefoil factor 2/porcine spasmolytic polypeptide in the rat. Gut 1998, 43, 240–247.
- Poulsen, S.S.; Thulesen, J.; Hartmann, B.; Kissow, H.; Nexø, E.; Thim, L. Injected TFF1 and TFF3 bind to TFF2-immunoreactive cells in the gastrointestinal tract in rats. Regul. Pept. 2003, 115, 91–99.
- Nakayama, J. Dual roles of gastric gland mucin-specific o-glycans in prevention of gastric cancer. Acta Histochem. Cytochem. 2014, 47, 1–9.
- Emidio, N.B.; Brierley, S.M.; Schroeder, C.I.; Muttenthaler, M. Structure, function, and therapeutic potential of the trefoil factor family in the gastrointestinal tract. ACS Pharmacol. Transl. Sci. 2020, 3, 583–597.
- Hoffmann, W. Trefoil factor family (TFF) peptides and chemokine receptors: A promising relationship. J. Med. Chem. 2009, 52, 6505–6510.
- Dubeykovskaya, Z.; Si, Y.; Chen, X.; Worthley, D.L.; Renz, B.W.; Urbanska, A.M.; Hayakawa, Y.; Xu, T.; Westphalen, C.B.; Dubeykovskiy, A.; et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat. Commun. 2016, 7, 10517.
- Porębska, N.; Poźniak, M.; Matynia, A.; Żukowska, D.; Zakrzewska, M.; Otlewski, J.; Opaliński, Ł. Galectins as modulators of receptor tyrosine kinases signaling in health and disease. Cytokine Growth Factor Rev. 2021, 60, 89–106.
- Soutto, M.; Chen, Z.; Bhat, A.A.; Wang, L.; Zhu, S.; Gomaa, A.; Bates, A.; Bhat, N.S.; Peng, D.; Belkhiri, A.; et al. Activation of STAT3 signaling is mediated by TFF1 silencing in gastric neoplasia. Nat. Commun. 2019, 10, 3039.
- Meanwatthana, J.; Majam, T. Interleukin-6 antagonists: Lessons from cytokine release syndrome to the therapeutic application in severe COVID-19 infection. J. Pharm. Pract. 2021, 08971900211000691.
- Emidio, N.B.; Hoffmann, W.; Brierley, S.; Muttenthaler, M. Trefoil factor family: Unresolved questions and clinical perspectives. Trends Biochem. Sci. 2019, 44, 387–390.
- Chen, R.-M.; Chiou, Y.-S.; Chong, Q.-Y.; Poh, H.-M.; Tan, T.-Z.; Zhang, M.-Y.; Ma, L.; Zhu, T.; Pandey, V.; Kumar, A.P.; et al. Pharmacological inhibition of TFF3 enhances sensitivity of CMS4 colorectal carcinoma to 5-fluorouracil through inhibition of p44/42 MAPK. Int. J. Mol. Sci. 2019, 20, 6215.
- Bolle, T.; Meyer, F.; Walcher, F.; Lohmann, C.; Jockenhövel, S.; Gries, T.; Hoffmann, W. Materials/biomaterials in clinical practice—A short review and current trends. Zentralbl. Chir. 2017, 142, 216–225.
