CWMFC is a novel technology that has been used for almost a decade for concurrent wastewater treatment and electricity generation in varying scopes of domestic, municipal, and industrial applications since its implementation in 2012. Its advantage of low-cost enhanced wastewater treatment and sustainable bioelectricity generation has gained considerable attention. Nevertheless, the overall efficiency of this novel technology is inclined by several operating factors and configuration strands, such as pH, sewage composition, organic loading, electrode material, filter media, electrogens, hydraulic retention time, and macrophytes. Here, we investigate the effect of the wetland plant component on the overall performance of CWMFCs. The macrophyte’s involvement in the oxygen input, nutrient uptake, and direct degradation of pollutants for the required treatment effect and bioelectricity production are discussed in more detail. The review identifies and compares planted and unplanted CWMFC with their efficiency on COD removal and electricity generation based on previous and recent studies.
- electricity generation
- wetland plants
- wastewater treatment
- microbial fuel cell
- constructed wetlands
1. Introduction
Over the decades, many wastewater treatment technologies have been employed to address the wastewater environmental menace. Wastewater treatment technologies, which consist of trickling filters, activated sludge, reverse osmosis, and membrane filters, are currently being used to treat all types of organic and toxic wastewater from industrial and municipal sources. However, they are not very productive, with regards to the cost and energy demand required in their operation [1]. It is projected that USD 2 trillion will be required in the U.S.A over the next 20 years to construct, operate, and maintain wastewater and drinking water facilities [2]. In addition to the current annual costs of USD 25 billion, around USD 45 billion is expected for wastewater infrastructure upgrades, with over half of operating expenditures aimed at aeration of wastewater. Power production measured here only for aeration could provide much-needed energy in the U.S.A from industrial wastewater alone [3]. According to Gude (2015), some of these conventional wastewater treatment systems require 0.3–0.6 kW∙h∙m −3 for treatment, whereas inherent in the same wastewater is energy that is equivalent to 10 times that needed for treatment [4]. Hence, the concept of generating electrical energy from the inherent chemical energy (organic matter) in wastewater during the treatment process will help offset the financial burden of treatment and provide access to clean water throughout the world, which would be highly recognized as sustainable [5][6].
In 1911, Michael C. Potter experimented and put forward the first microbial electrochemical technology (MET) and bio-electrochemical system (BES), established as microbial fuel cells (MFC), as a sustainable biotechnology [7][8]. A microbial fuel cell is an innovative wastewater treatment technology that uses electrochemical active bacteria (EAB) as a biocatalyst to transform the chemical energy inherent in sewage directly into electrical production without any environmental footprint [1][8]. MFCs use wastewater as a feed substrate for EABs to produce bio-electricity, while concurrently treating waste [1]. According to, Singh et al. [1], MFC as a technology holds great potential for a clean and green energy environment.
Constructed wetlands (CWs), on the other hand, are bio-physically assembled systems designed and built to take advantage of natural processes and interactions between wetland flora, soils, and associated microbial species to help regenerate wastewater [9][10]. Wastewater from a wide variety of sources, such as municipal, agricultural, or industrial wastewater, are treated by CWs [11]. They are easy to maintain and operate and can remediate many of the persistent pollutants that occur in conventional wastewaters into harmless by-products [12]. As a result, they have emerged as a substitute to traditional intensified systems for wastewater treatment [13][14]. A decade ago, researchers discovered that the embedded redox gradients, which naturally exist in wetlands, are highly compatible with the settings in microbial fuel cells, i.e., anaerobic zone in the inner–lower region and aerobic region at the air–water interface [15]. This connection makes their incorporation very plausible by creating a synergy between these two technologies for enhanced wastewater regeneration and bioenergy generation [16].
2. Configuration of CWMFC
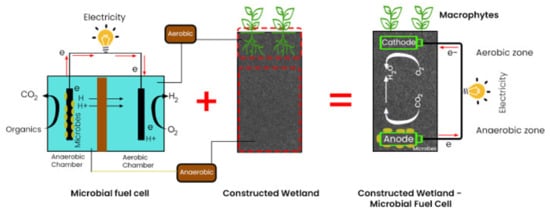
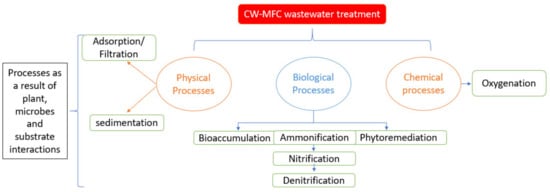
3. Role of Macrophyte in CW-MFC Contaminant Removal
| Macrophyte | Initial COD (mg/L) | COD Removal (%) | HRT (hr) | Max. Power | Author |
|---|---|---|---|---|---|
| Canna indica | 1500 | 74.9 | 96 | 15.7 mW∙m−2 | [16] |
| Phragmites australis | 1058 | 76.5 | N. A | 9.4 mW∙m−2 | [45] |
| Ipomoea aquatica | 180 | 86 | 72 | 0.302 W∙m−3 | [28] |
| Phragmites australis | 250 | 80–100 | N. A | 0.15 mW∙m−2 | [46] |
| Ipomoea aquatica | 193–205 | 94.8 | 48 | 12.42 mW∙m−2 | [43] |
| Ipomoea aquatica | 300 | 72.5 | 72 | 0.852 W∙m−3 | [47] |
| Phragmites australis | 411–854 | 64 | N. A | 0.268 W∙m−3 | [25] |
| Typha latifolia | 314.8 | 100 | N. A | 6.12 mW∙m−2 | [48] |
| Phragmites australis | 583 | 64 | N. A | 0.276 W∙m−3 | [26] |
| Taifa latifolia | 624 | 99 | 24 | 93 mW∙m−3 | [49] |
| Phragmite australis | 323 | 60.6 | 62.4 | 131 mW∙m−2 | [50] |
| Elodea nuttallii | 643 | 97–98 | 24 | 184.75 mW∙m−3 | [34] |
| Canna indica | -- | 78.71 | 72 | 31.04 mW∙m−3 | [51] |
| Phragmites australis | 200 | 90.45 | 48 | 0.20 W∙m−3 | [52] |
| Phragmites australis | -- | 82 | 72 | 3714 mW∙m−2 | [53] |
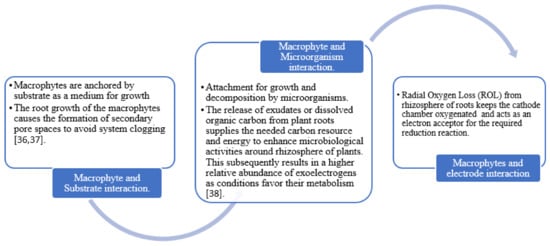
In a CWMFC configuration, the appropriate selection of macrophytes is crucial for the system’s success. The macrophyte component is one of the most conspicuous and versatile parts of the CWMFC bio-electrochemical system. The type of wetland plant installed in CWMFCs has some unique properties that make them play such an essential role in the contaminant removal processes and bioelectricity generation [54][55]. Therefore, a thorough selection of the type of macrophyte to be used is hugely imperative to the system’s success. This decision can either enhance or retard CWMFCs efficiency significantly. Hence, an appropriate selection of wetland plants must be based on some unique characteristics, such as:
Table 2: Characteristics of Macrophytes and their relevance in CWMFC.
|
Macrophyte properties |
Relevance in CWMFC |
|
rapid growth and high biomass production |
For winter insulation in cold and temperate regions, and particularly for the removal of nutrients by harvesting as nutrients are absorbed by macrophytes to build their biomass [55]. In addition, according to Yang et al. [56], species with high biomass production in CWMFC enhances the cell voltage and reduces the internal resistance of the system which often result in higher bioenergy production. |
|
good natural adaptation to the local climate |
Native species should be best preferred. According to Sierra (2017), CWMFC plants are selected based on the region's most common aquatic plants [27]. Oodally et al., (2019), concluded that native species are best preferred due to their local climate adaptability. In their experimentation, the most common aquatic plants in the region showed improved performance in CWMFC than exotic species [47].
|
|
good root development |
To provide a substrate for attached bacteria and oxygenation [55]. Also, the root development or maturity of the wetland plant affects oxygen release. In a sediment microbial fuel cell (SMFC) with wetland plant experiments conducted by Chen et al., (2012), their investigation has shown that young roots could excrete more oxygen than mature or aging species. Similarly, Stolzenberg et al. [57] also observed that plant species with good root development produced better oxygen which presented the highest voltage value than plants with smaller poor root systems. In addition, Mosqsud et al. operated a series of 6-CWMFC using Oriza sativa species. In their experimentation, they observed a reduction in power production as plants attained maturation. This was mainly because the maturation of the plant affected both oxygen release and exudate production. This signifies that the maturity of the root and its development is an essential factor in wetland plant selection [58].
|
|
High oxygen transfer capacity |
Oxygen transfer capacity from the roots creates an aerobic environment. Due to the great diversity of flora, different species have different radial oxygen loss (ROL) [19]. |
|
nutrient absorption capacity |
High nutrient absorption capacity helps in the effective removal of contaminants from the system. Species with high NAC use absorbed nutrients as a resource for their metabolism and growth [33][59]. |
|
adaptation and ease of propagation |
The ease in getting seedlings, seeds, or vegetative propagules must be well considered to ensure system sustainability. |
|
Good Rhizodeposition; release of carbon sources as rhizodeposits from plant roots. |
Rhizodeposition supports the growth and activities of microorganisms associated with bioelectricity production. |
|
C4 Plants |
The photosynthetic activity of plants is categorized into 3-phases: C3, C4, and CAM. In terms of oxygen production and CO2 fixation, plants in each category have different photosynthetic pathways. Plants in the group of C4 are those with advanced photosynthetic activity than plants in C3 and CAM groups. Consequently, because they have a higher conversion rate of solar energy into bioelectricity, it is suggested to integrate C4 plants [60]. |
These factors should be primarily considered in the appropriate selection of macrophytes for CWMFC. Nevertheless, owing to the wide variety of aquatic flora, further investigation is needed to evaluate and select plant species with potentials for CWMFC for simultaneous wastewater regeneration and bioelectricity production.
Macrophytes, particularly emergent plants, can cause substantial water loss in CWMFC through evapotranspiration. As the volume of wastewater flowing through the system decreases due to water loss, the treatment efficiency in CWMFCs could be affected significantly when the evapotranspiration rate exceeds 2.5 mm/d [34]. Also, in the absence of light, plant cells and microorganism respiration will consume O2. Hence, the DO level in the reactor was reduced as DO consumption was more than production. The plant's photosynthesis and respiration altered the reactor's oxygen dynamics, ultimately leading to voltage fluctuations [61]. Therefore, macrophyte species that can help overcome this setback will be highly recommended.
This entry is adapted from the peer-reviewed paper 10.3390/app11167454
References
- Singh, H.M.; Pathak, A.K.; Chopra, K.; Tyagi, V.V.; Anand, S.; Kothari, R. Microbial fuel cells: A sustainable solution for bioelectricity generation and wastewater treatment. Biofuels 2019, 10, 11–31.
- Glover, H.; Guz, E.; Hanewall, C.; Hollander, A.; Kocian, A. Alternative Financing of Water and Wastewater Infrastructure in Rural Communities; United States Department of Agriculture, Rural Development: Washington, DC, USA; Maxwell School of Syracuse University: Syracuse, NY, USA, 2005.
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of Electricity during Wastewater Treatment Using a Single Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 2281–2285.
- Gude, V.G. Energy and water autarky of wastewater treatment and power generation systems. Renew. Sustain. Energy Rev. 2015, 45, 52–68.
- Virdis, B.; Freguia, S.; Rozendal, R.A.; Rabaey, K.; Yuan, Z.; Keller, J. Microbial Fuel Cells. In Treatise on Water Science; Elsevier: Amsterdam, The Netherlands, 2011; Volume 4, pp. 641–665.
- Das, D. Microbial Fuel Cell—A Bioelectrochemical System that Converts Waste to Watts; Springer: Berlin/Heidelberg, Germany, 2018; p. 20. ISBN 978-3-319-66792-8.
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. B Biol. Sci. 1911, 84, 260–276.
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008.
- Vymazal, J. Constructed Wetlands for Wastewater Treatment. Water 2010, 2, 530–549.
- Yang, E.; Chae, K.-J.; Choi, M.-J.; He, Z.; Kim, I.S. Critical review of bioelectrochemical systems integrated with membrane-based technologies for desalination, energy self-sufficiency, and high-efficiency water and wastewater treatment. Desalination 2019, 452, 40–67.
- Vidal, C.C. Constructed Wetland Microbial Fuel Cells: Electricity Generation, Treatment Efficiency Improvement, COD Bioindication and Clogging Assessment. Ph.D. Thesis, Universitat Politecnica de Catalunya, Barcelona, Spain, 2017.
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601.
- Scholz, M.; Lee, B. Constructed wetlands: A review. Int. J. Environ. Stud. 2005, 62, 421–447.
- Yadav, A.K.; Srivastava, P.; Kumar, N.; Abbassi, R.; Mishra, B.K. Constructed Wetland-Microbial Fuel Cell: An Emerging Integrated Technology for Potential Industrial Wastewater Treatment and Bio-Electricity Generation. Constr. Wetl. Ind. Wastewater Treat. 2018, 493–510.
- Wang, Y.; Zhao, Y.; Xu, L.; Wang, W.; Doherty, L.; Tang, C.; Ren, B.; Zhao, J. Constructed wetland integrated microbial fuel cell system: Looking back, moving forward. Water Sci. Technol. 2017, 76, 471–477.
- Yadav, A.K.; Dash, P.; Mohanty, A.; Abbassi, R.; Mishra, B.K. Performance assessment of innovative constructed wetland-microbial fuel cell for electricity production and dye removal. Ecol. Eng. 2012, 47, 126–131.
- Sierra, M.A.; Esteve Núñez, A.; Salas Rodriguez, J.J. Integrating Microbial Electrochemical Systems in Constructed Wetlands, a New Paradigm for Treating Wastewater in Small Communities. Ph.D. Thesis, Universidad de Alcalá, Madrid, Spain, 2017; pp. 100–165.
- Yang, Y.; Zhao, Y.; Liu, R.; Morgan, D. Global development of various emerged substrates utilized in constructed wetlands. Bioresour. Technol. 2018, 261, 441–452.
- Yan, D.; Song, X.; Weng, B.; Yu, Z.; Bi, W.; Wang, J. Bioelectricity generation from air-cathode microbial fuel cell connected to constructed wetland. Water Sci. Technol. 2018, 78, 1990–1996.
- Fang, Z.; Cheng, S.; Wang, H.; Cao, X.; Li, X. Feasibility study of simultaneous azo dye decolorization and bioelectricity generation by microbial fuel cell-coupled constructed wetland: Substrate effects. RSC Adv. 2017, 7, 16542–16552.
- Srivastava, P.; Yadav, A.K.; Mishra, B.K. The effects of microbial fuel cell integration into constructed wetland on the performance of constructed wetland. Bioresour. Technol. 2015, 195, 223–230.
- Araneda, I.; Tapia, N.F.; Allende, K.L.; Vargas, I.T. Constructed Wetland-Microbial Fuel Cells for Sustainable Greywater Treatment. Water 2018, 10, 940.
- Kalathil, S.; Patil, S.A.; Pant, D. Microbial Fuel Cells: Electrode Materials; Elsevier Inc.: Amsterdam, The Netherlands, 2018.
- Shi, Y.; Yang, X.; Ning, X.; Yang, Q. Research progress of microbial fuel cell and constructed wetland coupling system. IOP Conf. Ser. Earth Environ. Sci. 2018, 199, 052014.
- Doherty, L.; Zhao, X.; Zhao, Y.; Wang, W. The effects of electrode spacing and flow direction on the performance of microbial fuel cell-constructed wetland. Ecol. Eng. 2015, 79, 8–14.
- Doherty, L.; Zhao, Y.; Zhao, X.; Wang, W. Nutrient and organics removal from swine slurry with simultaneous electricity generation in an alum sludge-based constructed wetland incorporating microbial fuel cell technology. Chem. Eng. J. 2015, 266, 74–81.
- Jingyu, H.; Miwornunyuie, N.; Ewusi-Mensah, D.; Koomson, D. Assessing the factors influencing the performance of constructed wetland–microbial fuel cell integration. Water Sci. Technol. 2020, 81, 631–643.
- Fang, Z.; Song, H.-L.; Cang, N.; Li, X.-N. Performance of microbial fuel cell coupled constructed wetland system for decolorization of azo dye and bioelectricity generation. Bioresour. Technol. 2013, 144, 165–171.
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Müller, R.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117.
- Wang, L.; Li, Y.; Li, X.; Han, B. A labscale study on constructed wetland microbial fuel cell. Acta Sci. Circumstantiae 2017, 37, 3656–3663.
- Zhai, X.; Piwpuan, N.; Arias, C.A.; Headley, T.; Brix, H. Can root exudates from emergent wetland plants fuel denitrification in subsurface flow constructed wetland systems? Ecol. Eng. 2013, 61, 555–563.
- Guang, L.; Koomson, D.A.; Jingyu, H.; Ewusi-Mensah, D.; Miwornunyuie, N.; Miwornunyuie, N. Performance of Exoelectrogenic Bacteria Used in Microbial Desalination Cell Technology. Int. J. Environ. Res. Public Health 2020, 17, 1121.
- Greenway, M. The Role of Macrophytes in Nutrient Removal using Constructed Wetlands. Environ. Bioremediat. Technol. 2007, 331–351.
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Dahalan, F.A.; Lehl, H.; Thung, W.-E.; Nordin, N. Role of macrophyte and effect of supplementary aeration in up-flow constructed wetland-microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2017, 224, 265–275.
- Srivastava, P.; Yadav, A.K.; Garaniya, V.; Abbassi, R. Constructed Wetland Coupled Microbial Fuel Cell Technology. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1021–1036.
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65.
- Wang, Q.; Hu, Y.; Xie, H.; Yang, Z. Constructed Wetlands: A Review on the Role of Radial Oxygen Loss in the Rhizosphere by Macrophytes. Water 2018, 10, 678.
- Guadarrama-Pérez, O.; Gutiérrez-Macías, T.; García-Sánchez, L.; Guadarrama-Pérez, V.H.; Estrada-Arriaga, E.B. Recent advances in constructed wetland-microbial fuel cells for simultaneous bioelectricity production and wastewater treatment: A review. Int. J. Energy Res. 2019, 43, 5106–5127.
- Wen, H.; Zhu, H.; Yan, B.; Shutes, B.; Yu, X.; Cheng, R.; Chen, X.; Wang, X. Constructed wetlands integrated with microbial fuel cells for COD and nitrogen removal affected by plant and circuit operation mode. Environ. Sci. Pollut. Res. 2021, 28, 3008–3018.
- Chiranjeevi, P.; Yeruva, D.K.; Kumar, A.K.; Mohan, S.V.; Varjani, S. Plant-microbial fuel cell technology. Microb. Electrochem. Technol. 2019, 549–564.
- Brix, H.; Schierup, H.H. The Use of Aquatic Macrophytes in Water-Pollution Control. Ambio 1989, 28, 100–107.
- Wen, H.; Zhu, H.; Yan, B.; Xu, Y.; Shutes, B. Treatment of typical antibiotics in constructed wetlands integrated with microbial fuel cells: Roles of plant and circuit operation mode. Chemosphere 2020, 250, 126252.
- Liu, S.; Song, H.; Li, X.; Yang, F. Power Generation Enhancement by Utilizing Plant Photosynthate in Microbial Fuel Cell Coupled Constructed Wetland System. Int. J. Photoenergy 2013, 2013, 172010.
- Oodally, A.; Gulamhussein, M.; Randall, D.G. Investigating the performance of constructed wetland microbial fuel cells using three indigenous South African wetland plants. J. Water Process Eng. 2019, 32, 100930.
- Zhao, Y.; Collum, S.; Phelan, M.; Goodbody, T.; Doherty, L.; Hu, Y. Preliminary investigation of constructed wetland incorporating microbial fuel cell: Batch and continuous flow trials. Chem. Eng. J. 2013, 229, 364–370.
- Villaseñor, J.; Capilla, P.; Rodrigo, M.A.; Cañizares, P.; Fernández, F. Operation of a horizontal subsurface flow constructed wetland–microbial fuel cell treating wastewater under different organic loading rates. Water Res. 2013, 47, 6731–6738.
- Fang, Z.; Song, H.-L.; Cang, N.; Li, X.-N. Electricity production from Azo dye wastewater using a microbial fuel cell coupled constructed wetland operating under different operating conditions. Biosens. Bioelectron. 2015, 68, 135–141.
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Lehl, H.; Thung, W.-E. Hybrid system up-flow constructed wetland integrated with microbial fuel cell for simultaneous wastewater treatment and electricity generation. Bioresour. Technol. 2015, 186, 270–275.
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Dahalan, F.A.; Lehl, H.; Thung, W.-E. Synergistic effect of up-flow constructed wetland and microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2016, 203, 190–197.
- Corbella, C.; Garfí, M.; Puigagut, J. Long-term assessment of best cathode position to maximise microbial fuel cell performance in horizontal subsurface flow constructed wetlands. Sci. Total Environ. 2016, 563–564, 448–455.
- Srivastava, P.; Dwivedi, S.; Kumar, N.; Abbassi, R.; Garaniya, V.; Yadav, A.K. Performance assessment of aeration and radial oxygen loss assisted cathode based integrated constructed wetland-microbial fuel cell systems. Bioresour. Technol. 2017, 244, 1178–1182.
- Song, H.; Zhang, S.; Long, X.; Yang, X.; Li, H.; Xiang, W. Optimization of Bioelectricity Generation in Constructed Wetland-Coupled Microbial Fuel cell Systems. Water 2017, 9, 185.
- Xu, F.; Cao, F.-Q.; Kong, Q.; Zhou, L.-L.; Yuan, Q.; Zhu, Y.-J.; Wang, Q.; Du, Y.-D.; Wang, Z.-D. Electricity production and evolution of microbial community in the constructed wetland-microbial fuel cell. Chem. Eng. J. 2018, 339, 479–486.
- Villaseñor, J.; Capilla, P.; Rodrigo, M.A.; Cañizares, P.; Fernández, F. Operation of a horizontal subsurface flow constructed wetland–microbial fuel cell treating wastewater under different organic loading rates. Water Res. 2013, 47, 6731–6738.
- Wang, J.; Song, X.; Wang, Y.; Bai, J.; Li, M.; Dong, G.; Lin, F.; Lv, Y.; Yan, D. Bioenergy generation and rhizodegradation as affected by microbial community distribution in a coupled constructed wetland-microbial fuel cell system associated with three macrophytes. Sci. Total Environ. 2017, 607–608, 53–62.
- Białowiec, A.; Albuquerque, A.; Randerson, P.F. The influence of evapotranspiration on vertical flow subsurface constructed wetland performance. Ecol. Eng. 2014, 67, 89–94.
- Liu, F.; Sun, L.; Wan, J.; Shen, L.; Yu, Y.; Hu, L.; Zhou, Y. Performance of different macrophytes in the decontamination of and electricity generation from swine wastewater via an integrated constructed wetland-microbial fuel cell process. J. Environ. Sci. 2020, 89, 252–263.
- Chen, Z.; Huang, Y.-C.; Liang, J.-H.; Zhao, F.; Zhu, Y.-G. A novel sediment microbial fuel cell with a biocathode in the rice rhizosphere. Bioresour. Technol. 2012, 108, 55–59.
- Vymazal, J. Plants used in constructed wetlands with horizontal subsurface flow: A review. Hydrobiologia 2011, 674, 133–156.
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Total Environ. 2009, 407, 3923–3930.
- Xu, L.; Zhao, Y.; Doherty, L.; Hu, Y.; Hao, X. The integrated processes for wastewater treatment based on the principle of microbial fuel cells: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 60–91.
