Exosomes are a type of extracellular vesicle (EV) of endocytic origin that are released by virtually all cells in multicellular organisms and carry out important intercellular communication functions through the transfer of their biomolecular cargo, which includes lipids, proteins, nucleic acids and metabolites, between the producing and the recipient/target cells [1]. Exosomes produced by cancer cells have been shown to influence many processes related to cancer progression and metastasis, such as tumor cell proliferation and invasion, angiogenesis, tumor microenvironment promotion and remodelling, chemotherapy resistance, and immune suppression (recently reviewed in [2,3,4]).
1. Introduction
Colorectal cancer (CRC) has a high incidence and is a major cause of cancer-related mortality worldwide, with approximately 25% of patients developing peritoneal metastasis, a condition associated with a bleak prognosis as current treatments are less effective in these patients [5]. The CRC peritoneal dissemination cascade involves sequential shedding of cancer cells from the primary colonic or rectal tumor, their transport through the peritoneal cavity following the physiological flow of peritoneal fluid, their adhesion to the peritoneal mesothelial cells (PMCs) that form a barrier lining all peritoneal organs, and the subsequent invasion of cancer cells through this mesothelial cell barrier and the underlying stroma to establish new metastatic foci in the peritoneum, omentum and bowel serosa. In addition to acting as a barrier, PMCs also play an active role in the pathogenesis of peritoneal metastasis by differentiating into cancer-associated fibroblasts (CAFs) through a process termed Mesothelial-to-Mesenchymal Transition (MMT), which fuels peritoneal metastasis and immune evasion (reviewed in [5]).
In epithelial ovarian cancer, which predominantly disseminates via peritoneal metastasis, exosomes produced by ovarian cancer cells favor different steps of the peritoneal dissemination cascade and have therefore been recognized to play crucial roles in the pathogenesis of the disease [6,7]. Of particular relevance, cancer-derived exosomes in malignant ascites interact with cancer cells promoting their survival, migration and invasion capacities, and with PMCs inducing their apoptosis, disrupting the mesothelial barrier and reprogramming them into CAFs through MMT. For CRC, however, current knowledge about the roles played by exosomes in the pathogenesis and peritoneal metastasis cascade is sparse. At the same time, the molecules that mediate the interactions between cancer-derived exosomes and their target PMCs and tumor cells mostly remain obscure.
Previously we employed the human colorectal adenocarcinoma Colo-320 cell line [8] as a useful model to study cancer-related processes such as tumorigenesis, metastasis, and tumor cell adhesion, as well as the functions and interplay between tetraspanins and cell adhesion molecules [9,10,11,12,13,14]. Colo-320 cells possess the distinctive feature of lacking endogenous expression of tetraspanin CD9 while they abundantly express integrin α5β1 but not many other members of the β1 integrin subfamily. CD9 is a widely distributed tetraspanin that associates with other transmembrane proteins, including integrins and ADAM metalloproteases in TEMs (Tetraspanin-Enriched Microdomains) (reviewed in [15]). CD9 is involved in cell adhesion, motility, sperm–egg fusion, tumorigenesis and metastasis and an inverse correlation between expression of CD9 and metastatic potential and patient survival rate has been established in many types of cancer, including CRC (reviewed in [16]).
Several reports indicate that integrin α5β1 can engage in cis (on the same cell) and trans (on different cells) interactions with the distintegrin (Dis) domain of the metalloproteinase ADAM17/TACE [14,15,17,18]. Recent study showed that the tetraspanin CD9 negatively regulated integrin α5β1-mediated adhesion of cancer cells both to its canonical ligand fibronectin and to ADAM17 as a novel ligand [14,15]. Furthermore, it has reported that CD9 interacts directly with ADAM17 on the cell surface and regulates negatively both the sheddase and adhesive activities of this metalloproteinase [12,13,14,15,19,20,21]. Therefore, we decided to investigate whether integrin α5β1 and ADAM17 interaction and the regulatory effects of CD9 also played a role in mediating the binding of exosomes and their uptake by cancer or mesothelial recipient cells, which could bear relevance during the peritoneal dissemination of colorectal carcinomas.
We report here that the interaction between integrin α5β1 on colorectal carcinoma cells and on PMCs and its ligand ADAM17 on exosomes mediates the binding and uptake of cancer-derived exosomes. Furthermore, this process was negatively regulated by the expression of tetraspanin CD9 on the surface of exosomes.
2. Results
2.1. Characterization of EVs Derived from Colo-320, Colo-320/CD9 and Colo-320/ADAM17-KO Human Adenocarcinoma Cells
The generation of two stable lines derived from parental human colorectal adenocarcinoma Colo-320 cells: Colo-320/CD9, expressing high levels of cell surface CD9 after stable transfection; and Colo-320/ADAM17-KO, lacking expression of the metalloproteinase ADAM17/TACE after specific CRISPR/Cas9 knock-out of this enzyme [
14] (see also
supplementary material). EVs were isolated from FBS-free conditioned media of Colo-320, Colo-320/CD9 and Colo-320/ADAM17-KO cells, by a protocol that included two sequential centrifugations, first at 300×
g to discard whole cells and large cellular debris, and at 10,000×
g to discard the pellet containing particulate material and larger extracellular vesicles such as microvesicles. The resultant supernatants were [
20] concentrated by tangential flow and spin filtrations and EVs purified by size−exclusion chromatography (SEC) fractionation in a 30 mL Sepharose Fast-Flow 4 column (
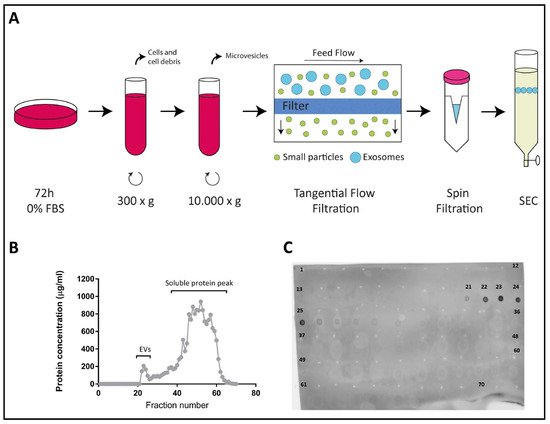
Figure 1A and described in detail in Materials and Methods). Typically, a total of 70 fractions (0.5 mL each) were collected from each sample and their protein content was quantified using the Micro BCA Protein Assay Kit.
Figure 1B shows a representative graph of the protein content of all collected fractions with two clearly distinguishable peaks: a small first peak, which corresponded to the elution of EVs, typically comprising fractions 21–25, and a much larger second peak (fractions 26–70) corresponding to the elution of free soluble protein. The exosomal content of the fractions in the first peak was confirmed by dot blot analysis using an antibody against the tetraspanin CD81, a classical EV marker (
Figure 1C) [
22,
23].
Figure 1. Isolation of exosomes from Colo-320 cells. (A) Experimental procedure for isolation of exosomes from conditioned medium of Colo-320 cells. Cells were cultured in serum-free conditions for 72 h before collection of the medium, which was then sequentially centrifuged at 300× g to remove cells and large debris and at 10,000× g to remove larger EVs such as microvesicles. Medium was then concentrated by tangential flow filtration and spin filtration to a final 1.0 mL volume. Exosomes were finally isolated by size exclusion chromatography (SEC). (B) A representative SEC elution profile showing the protein concentration of the 70 fractions (0.5 mL each) typically collected. (C) Dot blot showing the fractions containing exosomes, based on the presence of the exosomal marker CD81.
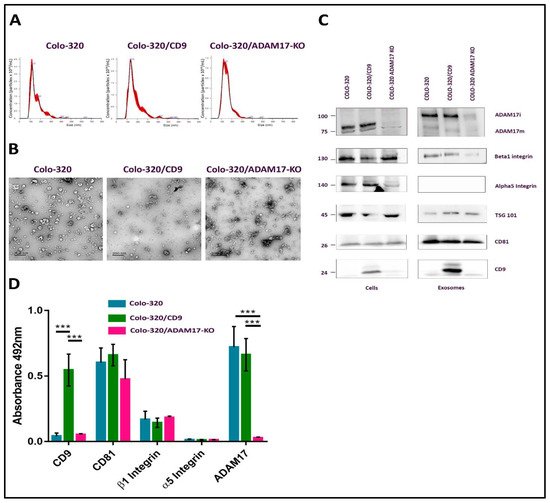
The CD81-positive fractions of the first peak (fractions 21–25) were pooled and the size distribution and concentration of EVs in the resulting samples were determined by Nano Tracking Analysis (NTA) (
Figure 2A). Typically, EV concentration in pooled samples was around 1–4 × 10
10 particles/mL, and the diameter of the particles fell within the range of 70–150 nm, as expected for exosomes. The round cup-like morphology of these particles, as visualized by transmission electron microscopy after negative staining, also corresponded to exosomes (
Figure 2B). The presence of CD81, CD9, TSG101, α5 integrin, β1 integrin and ADAM17 in exosome samples and in the producing cells was detected by western blotting, using specific antibodies (
Figure 2C). In addition, the content of CD81, CD9, α5 integrin, β1 integrin and ADAM17 in these exosome samples was also quantified after their immobilization on plastic wells (5 μg of total EV protein/well) by an indirect ELISA assay using specific primary antibodies to these proteins and HRP-conjugated secondary antibody (
Figure 2D). CD81 and TSG101, which are considered
bona fide exosomal markers [
21], were detected abundantly in the exosomes derived from the three cell lines. As expected, CD9 was detected only in exosomes derived from the CD9-positive Colo-320/CD9 cells, but not in exosomes derived from the CD9-negative Colo-320 or Colo-320/ADAM17-KO cells. Surprisingly, while the integrin β1 subunit was readily detected both in cell and EV lysates by western blot (
Figure 2C), on the plasma membrane of the three cell lines (assessed by flow cytometry,
Supplementary Figure S1) and in the exosomes derived from them (assessed by western blot and ELISA,
Figure 2C,D), the integrin α5 subunit, despite being expressed abundantly in cell lysates (
Figure 2C) and on the surface of the three cell lines (
Supplementary Figure S1) could not be detected (either by WB or by ELISA) in the exosomes derived from any of them (
Figure 2C,D). These results could indicate that in these cells the integrin α5 was actively excluded from exosomes by a process of selective protein sorting.
Figure 2. Characterization of exosomes produced by Colo-320 cells. (A) NTA analysis of the number and size of particles (exosomes) produced by Colo-320, Colo-320/CD9 and Colo-320/ADAM17-KO. (B) Transmission electron microscopy of exosomes produced by Colo-320, Colo-320/CD9 and Colo-320/ADAM17-KO. Scale bars= 200 nm. (C) Western blot showing the expression of ADAM17, CD9, CD81, TSG101, integrin β1 and integrin α5 in Colo-320, Colo-320/CD9 and Colo-320/ADAM17-KO cells and in their respective exosomes. Blots are representative of three different experiments. (ADAM17i: the immature form of the enzyme that contains the prodomain; ADAM17m: the mature form without the prodomain). (D) Detection of CD9, CD81, integrin β, integrin α5 and ADAM17 by enzyme-linked immunosorbent assay (ELISA) on immobilized exosomes produced by Colo-320, Colo-320/CD9 and Colo-320/ADAM17-KO cells. Data correspond to the mean ± SEM of three different experiments, each performed in duplicates. Statistical analysis performed was two-way ANOVA *** p < 0.001.
2.2. Fibronectin Is Not the Ligand of Integrin α5β1 That Mediates Interactions of Exosomes with Colo-320 Cancer Cells
Several groups have reported that exosomes produced by different cell types, including cancer cells, are coated with the extracellular protein fibronectin, and that this coat plays important roles in the interaction between these EVs and recipient/target cells and in directing cell movement through the tissues [
24,
25,
26,
27,
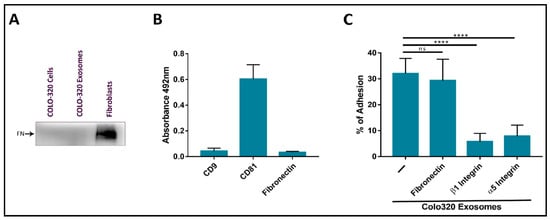
28]. The origin of the fibronectin found on exosomes can be endogenous from biosynthesis in the producing cells or exogenous from the bovine fibronectin present in FBS added to culture medium. In order to exclude exogenous fibronectin, we routinely deprived Colo-320 cancer cells of FBS for 72 h before exosome isolation. Following this protocol, we could not detect the presence of fibronectin on the surface of Colo-320 cancer cells by flow cytometry (
Supplementary Figure S1) or in cell lysates by western blot (
Figure 3A), which would indicate that these cells do not biosynthesize this protein. As a positive control, we detected abundant fibronectin on the surface of human fibroblasts cultured under the same conditions (72 h of FBS-deprivation) both by flow cytometry (
Supplementary Figure S2A) and in cell lysates by western blot (
Figure 3A). Therefore, as expected from the lack of fibronectin biosynthesis in these cells, this ECM protein could not be detected on the exosomes released by Colo-320 cells by either western blot (
Figure 3A) or by ELISA (
Figure 3B).
Figure 3. The adhesion of CRC Colo-320 cells to exosomes was specifically mediated by integrin α5β1 but not by fibronectin. (A) Western Blot of Colo-320 cells and their exosomes shows lack of expression of fibronectin. As a positive control, abundant fibronectin was detected in a fibroblasts lysate. Blots are representative of three different experiments. (B) Detection by enzyme-linked immunosorbent assay (ELISA) of CD9, CD81 and fibronectin on immobilized exosomes produced by Colo-320 cells. (C) Cell adhesion of Colo-320 cells to immobilized exosomes produced by these same cells was blocked by mAbs specific for integrin β1 or integrin α5, but not for fibronectin. The percentage of cells that remained adhered is indicated as mean ± SEM of four experiments, performed in duplicates. Statistical analysis performed was one-way ANOVA. **** p < 0.0001, ns: not statistically significant.
These adhesion assays had been previously employed by other groups to study the interactions of exosomes with recipient/target cells and led to the identification of some of the receptor and ligand molecules mediating such interactions [
25]. In these adhesion assays, around 35–40% of PMA-stimulated Colo-320 cells efficiently adhered to immobilized exosomes. Cell adhesion was almost completely abrogated by using blocking mAbs against the β1 (Lia1/2) or α5 (P1D6) chains of the fibronectin receptor integrin α5β1 (
Figure 3C), indicating that this adhesion molecule played a major role in these cell−exosome interactions. In contrast, a blocking anti human fibronectin mAb (HFN7.1), which completely knocked out the adhesion of Colo-320 cells to immobilized fibronectin (
Supplementary Figure S2B), did not significantly reduce cell adhesion to immobilized exosomes (
Figure 3C). These results concur with the observed lack of fibronectin content both in Colo-320 cells and in the exosomes derived from them and demonstrate that interactions of exosomes with Colo-320 cells were mediated by integrin α5β1 but not through the binding to its canonical ligand fibronectin.
2.3. Expression of CD9 Reduces Interactions between Cancer Cells and Exosomes Mediated by Cellular Integrin α5β1 and Exosomal ADAM17
The Disintegrin (Dis) domain of metalloproteinase ADAM17 has been reported by several groups, including ours, as a novel ligand for integrin α5β1 [
14,
17,
18,
29,
30]. Therefore, we investigated whether Dis-ADAM17 could be the integrin α5β1 ligand mediating the interactions of exosomes with Colo-320 cancer cells. As shown in
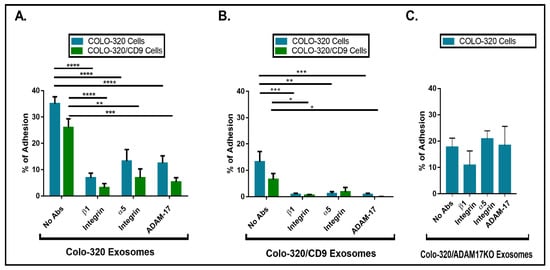
Figure 4A, a blocking antibody (mAb 2A10) specific for the Dis domain of ADAM17 [
14] significantly inhibited the adhesion of Colo-320 cells to immobilized exosomes. This effect was almost identical to the inhibition caused by blocking the integrin α5β1 with the P1D6 mAb. Taking into account that the only adhesion molecule described to interact in
trans with ADAM17 was the integrin α5β1, and also that the α5 chain of this integrin was abundantly expressed on Colo-320 cells (
Supplementary Figure S1) but not on exosomes (
Figure 2C,D), these results strongly indicate that the Dis domain of exosomal ADAM17 was the ligand being recognized by cellular integrin α5β1 during interactions of exosomes with Colo-320 cancer cells.
Figure 4. The presence of CD9 reduced cell adhesion to immobilized exosomes that was mediated by cellular integrin α5β1 and exosomal ADAM17. Cell adhesion of Colo-320 or Colo-320/CD9 to exosomes produced by Colo-320 cells (A), Colo-320/CD9 cells (B), and Colo-320/ADAM17-KO cells (C). Cells were stimulated with phorbol ester PMA (200 ng/mL) for 1 h, loaded with the fluorescent probe BCECF-AM and then allowed to adhere to immobilized exosomes (5 μg of exosomal protein per well) for 60 min at 37 °C under physiological conditions of Ca2+/Mg2+ (0.5 mM/1 mM, respectively). The percentage of cells that remained adhered is indicated as mean ± SEM of four experiments, performed in duplicates. Statistical analysis performed was two-way ANOVA. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
CD9 negatively regulates integrin α5β1-mediated cell adhesion to both its canonical ligand fibronectin and to the Dis domain of ADAM17 (Dis-ADAM17) [
14]. Therefore, we decided to assess whether CD9 could also regulate the
trans interaction of exosomal Dis-ADAM17 with cellular integrin α5β1. Firstly, we compared the capacity of exosomes either lacking (produced by parental Colo-320 cells) or expressing CD9 (produced by Colo-320/CD9 cells) to interact with Colo-320 and Colo-320/CD9 cells by allowing these cells to adhere to exosomes immobilized on plastic wells. The results of these assays (
Figure 4A,B) show that expression of CD9 on the cell surface resulted in a moderate but consistent reduction in their capacity to adhere to exosomes. More dramatic was the reduction of adhesion of Colo-320 and Colo-320/CD9 cells to exosomes when these EVs expressed CD9 on their surface, as cell adhesion dropped from 35% (
Figure 4A) to 13% (
Figure 4B). In all cases, cell adhesion was significantly inhibited by blocking mAbs specific for the β1 and α5 integrin subunits and for the Dis domain of ADAM17. These data establish that expression of CD9 on exosomes negatively regulates the capacity of Dis-ADAM17 to act as a ligand of integrin α5β1, and are in full agreement with the previously described effects of CD9 expression on ADAM17 on the surface of Colo-320 cells [
14].
The fact that expression of integrin α5β1 was high on the cell surface but minimal on the surface of exosomes pointed to a specific trans configuration, involving cellular integrin α5β1 and exosomal Dis-ADAM17 as molecules mediating these interactions of EVs with Colo-320 cells. To further prove this point, we assessed the adhesion of Colo-320 cells to immobilized exosomes lacking ADAM17 expression (isolated from Colo-320/ADAM17-KO cells). As shown in Figure 4C, adhesion of Colo-320 cells to ADAM17-KO exosomes (Colo-320/ADAM17-KO exosomes) was noticeably reduced compared to the adhesion to exosomes expressing ADAM17 (Colo-320 exosomes) (Figure 4A), demonstrating the crucial involvement of exosomal ADAM17 in these cell−exosome interactions. Importantly, a lack of ADAM17 expression on exosomes rendered these cell−exosome interactions completely resistant to the blocking effects of anti-α5 or anti-Dis-ADAM17 mAbs, confirming the involvement of ADAM17 on the exosomal side in such interactions and indicating that when exosomal ADAM17 was absent other adhesion molecule pairs may have taken over in mediating cell−exosome interactions.
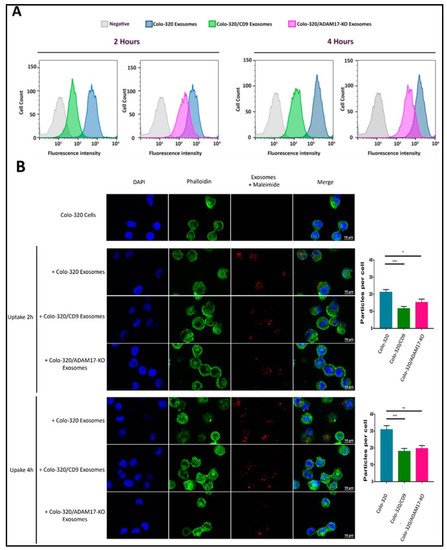
2.4. Uptake of Exosomes by Colo-320 Cancer Cells Depends on Exosomal ADAM17 and Is Inhibited by CD9
Our results showing that binding of cellular integrin α5β1 to its exosomal ligand Dis-ADAM17 mediated the initial interaction of exosomes with recipient cancer cells and that this process was negatively regulated by CD9 prompted us to investigate if these molecules also had an effect in the subsequent exosome uptake by these cells. For this purpose, we first employed a flow cytometry assay to quantitatively assess the uptake by Colo-320 cells of fluorescently labeled exosomes [
31].
Figure 5A shows that exosome uptake was markedly reduced after ADAM17 knock-out (Colo-320/ADAM17-KO exosomes) though not as pronounced as the reduction observed upon CD9 expression (Colo-320/CD9 exosomes). This reduction in the uptake was evident both after 2 h and 4 h of co-incubation of exosomes and cells. We also confirmed, using confocal microscopy, that exosomes derived from cells with deletion of ADAM17 or neo-expression of CD9 greatly reduced their uptake by recipient Colo-320 cancer cells (
Figure 5B). Although the limit of resolution of confocal microscopes is above the exosomal size, the fluorescent signal detected corresponded to aggregates of exosomes that accumulate in endosomal compartments after their uptake, as evidenced by their colocalization with the tetraspanin CD63 [
32].
Figure 5. Expression of exosomal CD9 and knocking-out ADAM17 expression in exosomes reduced the uptake of exosomes by Colo-320 recipient cells. (A) Flow cytometry quantitation of the uptake by Colo-320 recipient cells of fluorescently labelled exosomes produced by Colo-320, Colo-320/CD9 and Colo-320/ADAM17-KO cells, after 2 and 4 h or cell−exosome co-incubation. (B) Confocal microscopy images showing the uptake by Colo-320 recipient cells of the different exosomes as in (A). The bar graphs show the quantification of exosomal uptake by counting the particles internalized by at least 80 cells for each condition in three separate experiments. Statistical analysis performed was one-way ANOVA. * p < 0.05, ** p < 0.01 *** p < 0.001.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22189938