An effective analytical technique for biomass characterisation is inevitable for biomass utilisation in energy production. To improve biomass processing, various thermal conversion methods such as torrefaction, pyrolysis, combustion, hydrothermal liquefaction, and gasification have been widely used to improve biomass processing. Thermogravimetric analysers (TG) and gas chromatography (GC) are among the most fundamental analytical techniques utilised in biomass thermal analysis. Thus, GC and TG, in combination with MS, FTIR, or two-dimensional analysis, were used to examine the key parameters of biomass feedstock and increase the productivity of energy crops. We can also determine the optimal ratio for combining two separate biomass or coals during co-pyrolysis and co-gasification to achieve the best synergetic relationship.
- renewable energy
- thermochemical conversion
- thermal analysis
- thermogravimetric analysers
- gas chromatograph
- biomass and sustainability
Note: The entry will be online only after author check and submit it.
1. Introduction
Global energy demand has increased significantly over the last few decades due to the rising global population and economic prosperity. As a result, fossil fuel stocks are depleting, air pollution is on the rise, and greenhouse gas (GHG) emissions are increasing [1]. Many attempts have been made with an emphasis on overcoming these issues through the development of clean energy and alternative fuels. Even though renewable energy applications have grown rapidly in recent years, they remain limited due to high costs, low technology efficiency, and a limited supply of resources [2]. Biomass energy or bioenergy is one of the crucial challenges in meeting the requirements of substituted fossil fuels for reducing GHG emissions among the green energy and alternative fuels used in power generation [3]. The use of biomass will lower the country’s greenhouse gas emissions. When fossil fuels are burned, they release massive amounts of carbon dioxide into the atmosphere, which is carbon that would otherwise remain trapped underground. Heat and catalysts are used in the thermochemical processing of biomass to convert plant biomass into fuels, chemicals, or electric power. It has been used to treat crude oil in order to extract fossil fuels and various chemical products [4].
Biomass is one of the most adaptable, diverse, green, and renewable energy options available. Since the dawn of civilisation, it has been a primary source of energy for heating and cooking applications, especially in rural areas of developing countries [2]. Furthermore, in the present scenario, it contributes to a significant amount of overall electricity across the globe. Biomass can be harvested from several sources for long-term syngas processing, including timber wood and residuals, agricultural residues, aquaculture, biological by-products, drainage organic parts, and urban wastes [5]. The use of lignocellulosic biomass for energy production allows for the use of small-scale cogeneration units, lowering feedstock transportation costs, and making the energy system more robust to failures. To ensure continuous energy production and to handle a diverse biomass mix, the energy production equipment should be able to employ a variety of fuel types [6].
Biological and thermochemical processes are the most common methods for converting biomass into energy. Biological methods are widely used to produce hydrogen in biomass energy conversions. Figure 1 shows five distinct types of thermochemical processes. The thermochemical conversion of biomass is a viable option for overcoming the challenges associated with using biomass as a biofuel. The primary goal of thermochemical conversion processes is to reduce undesirable by-products by optimising process parameters. During the thermochemical conversion, heat and chemical processes are used to produce biofuel with high quality and densified energy content. Torrefaction, pyrolysis, combustion, liquefaction, and gasification are some of the thermochemical conversion processes that are used to transform lignocellulosic and non-lignocellulosic biomass into biofuels [7]. The research community and industry have shown an increased interest in the thermochemical conversion of biomass to transportation fuels. This green energy option has the potential to replace petroleum-derived fuels, benefiting many countries. Thermochemical biofuels are often produced locally and can help a country’s trade balance and national security [8].
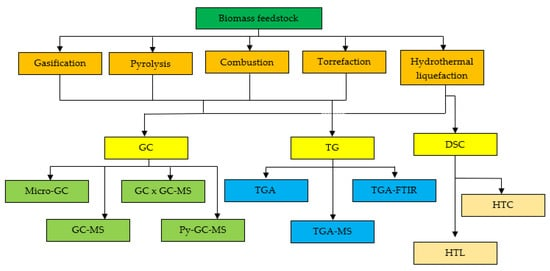
Figure 1. Schematic of analytic techniques for thermochemical conversion process.
This review focuses on recent advances in thermochemical conversion technology used to produce electricity, fuels of electricity, fuels, and chemicals from biomass. The combustion mechanism uses oxidation combustion reactions to produce heat from biomass. This review also focuses on thermochemical analytical methods because this is a proven science that has been used on a commercial scale. To gain a deeper understanding of the complexities and challenges of biomass conversion, biomass technology must first be defined [9]. Thermogravimetric analysis (TGA) is a thermal analytic technology that uses a thermogravimetric analyser (TG) to examine the mass shift of a sample with an increase in temperature and time [10]. TG has been an alternative way of studying the proximate analysis of biomass using reduced energy input to save money and time using ASTM requirements [11]. The functional groups and chemical structures of biomass and its products from thermochemical conversion can be identified primarily by observing changes in structure, heteroatomic functions, and mineral composition using Fourier transform infrared spectroscopy (FTIR) [12].
Along with other chromatographic methods, GC is critical in forensic research for separating substances of analytical significance. The idea behind chromatography is that molecules in a mixture are attracted to a surface or a solid, with the fluid stationary phase breaking apart and spinning with the aid of a mobile phase. Molecular characteristics such as adsorption, partition, and affinity, as well as variations in their molecular weights, are efficient in this separation process. Any components of the mixture will spend more time in the stationary phase and enter the chromatography system slowly, whereas others will enter the mobile phase rapidly and leave the system faster [13]. Chromatography is a technique for distinguishing molecules based on their distribution in a mobile and stationary phase. A liquid or gas may be used as the mobile phase. The stationary phase may be either a solid or a liquid, whereas a solid can hold a liquid stationary phase in place. The support or matrix is the solid that holds the liquid stationary phase in place [14]. Differential scanning calorimetry (DSC) is a thermo analytical technique that analyses a polymer’s thermal properties using a differential scanning calorimeter. This approach calculates the difference between the amount of heat required to raise the temperature of a sample and a reference as a function of temperature. Furthermore, this review is aimed to present thermal technologies and methods for analysing the composition of the mixture of products formed during the high-temperature decomposition of biomass feedstock. This review focuses on the products and composition of the biomass feedstock. This review also contains individual definitions and principles of TGA and GCMS, as well as their effect on the biomass thermochemical conversion process.
2. Thermochemical Conversion Process
The flexibility of feedstock used, product distribution (solid, liquid, and gas), and product upgrading are currently gaining researchers’ interest in thermochemical technology [15]. Generally, torrefaction, combustion, pyrolysis, gasification, and hydrothermal liquefaction are the five types of thermochemical conversion technologies for biomass [7]. Experiments on the thermochemical conversion of various biomass feedstocks at various operating conditions such as temperature, residence time, and pressure are being conducted in the lab and also on a pilot scale [16].
2.1. Gasification
Gasification is a chemical reaction that transforms carbonate elements into raw chemical substrates or gaseous fuels. The resulting gas mixture is known as synthesis gas, or syngas [17]. Using gasification process, a carbon-based feedstock is dissociated at high temperatures from 700 to 1500 °C under sub-stoichiometric conditions. The oxidising medium can be air, steam, pure oxygen, or a combination of these [18]. In gasification, biomass is heated to high temperatures exceeding 700 °C to produce syngas, which can be converted to liquid transportation fuels [19]. This method is based on a decade of experience with coal gasification. H2 and CO contribute roughly 50% of the energy in commodity gas, whereas CH4 and aromatic hydrocarbons contribute the remaining energy [20]. In the biomass method, the gasification of fossil fuels is common for the production of syngas (CO, H2, N2, and CO2), and a few hydrocarbon elements or compounds make up the majority of the syngas (CH4, C2H4 and C2H6 etc.) [21]. The gasification process produces heat when operates in an oxygen-deficient environment. Hence, a medium reaction, such as air, oxygen, subcritical steam, or certain gaseous mixtures, is required [22].
Co-gasification of coal and biomass supplies can be useful for a variety of reasons, including the fact that biomass has a high oxygen content, requiring less additional oxygen for the gasification system [23]. Compared to coal, biomass supplies have lower levels of ash, sulphur, and nitrogen. In the presence of an oxidising agent, co-gasification typically occurs at temperatures above 800 °C. The temperature needed for gasification is typically achieved by directly or indirectly heating the gasifier using strand and oxidising agents. The massive cellulosic and lignin molecules in the biomass degrade lighter molecules, which are then converted into gases such as CO, H2, CH4 and lighter hydrocarbons, as well as ash, char, tar, and small impurities [24]. The gasification process is highly dependent on operational parameters. The process involves various mechanisms such as biomass drying, pyrolysis, combustion, and reduction, all of which must be carried out under optimal conditions to achieve the desired end-product efficiency.
The gasification process involves the production of tar for syngas generation. A viable option for removing tar from gas products is catalytic steam reforming of tar into syngas [25]. Supported Ni-based catalysts are effective for catalytic reforming of biomass tar, which has the potential to produce syngas and be further synthesised by the Fischer-Tropsch reaction [26]. In a gasifier, a reaction kinetics model of hydrogen generation by biomass steam gasification with calcium oxide as sorbent was created. The biomass gasification process is influenced by the performance of each reaction represented in the model. The waster-gas shift reaction and methane steam are the two major processes that led to H2 generation. The findings revealed that the molar fraction of H2O increased from 0.065 to 0.83, whereas the molar fraction of CO2 decreased from 0.31 to 0.09 [27]. The char represented by x terms from gasification conversion rate is stated in Equation (1).
x=w0−wtw0−wf×100%(1)
where w0 is the sample’s initial mass, wt is the sample’s mass over time, and wf is the sample’s final mass. A well-established data analysis approach was used to investigate the reaction mechanism involved in a heat conversion process. The general response rate (dx/dt) is considered to be a function of the conversion (x) and a rate constant (k) and it can be written as Equation (2).
dxdt=kf (x)(2)
The plausible model of the reaction is denoted by f(x), The Arrhenius equation could be used to define the temperature-dependent gasification reaction rate constant (k) as represented in Equation (3).
k = A exp (−ERT)(3)
where A is the pre-exponential factor (min−1), E is the activation energy (kJ/mol), T is the absolute temperature (K), and R is the gas constant (8.314 J/(mol·K) [28]. To recover additional energy, most commercial gasification plants that handle municipal solid waste-derived feedstock use a secondary combustion chamber to burn the syngas and recover energy from a steam circuit. Another important by-product of gasification is solid leftovers of non-combustible materials containing a modest quantity of carbon. At various phases of the gasification process, high-temperature plasma gasification methods are also used. This plasma technology can generate tar-free, pure syngas, and the ash may be fused into glassy or vitreous residue [29]. The energetic efficiency (ηex) is the performance criterion used in the process performance condition. It is defined as the proportion of lucrative energy outputs flowing from the gasifier to the necessary energy input flow [30]:
ηex=£xgas+£xloss+£xtar+£xchar£xbiomass+£xsteam(4)
where £xgas, £xtar, £xchar, £xbiomass, and £xsteam are the loss energy flow and energy flow of gas, tar, char, biomass, and steam, respectively. Entropy creation, heat and mass transfers, and irreversibility of chemical reactions all result in a loss. The first and second thermodynamic laws must be followed in the gasification process. As a result of the second law, it is obtained by the following expression:
∑R£x−∑P£x=I(5)
where £x denotes energy and I denotes irreversibility, and it denotes the internal energy lost as a result of material quality deterioration and energy dissipation [31].
2.2. Pyrolysis
Pyrolysis is the thermochemical conversion of biomass by thermal movement into volatiles, steam, and a mixture of liquid compounds in an inert environment [32]. Organic feedstocks will be pyrolysed to yield three products: a liquid, a syngas, and a solid. During pyrolysis, natural polymeric components are broken down into volatile vapours comprising O- and H- containing forms, resulting in bio-oil. These gases can be condensed into bio-oil or recycled later in the pyrolysis process to help with energy requirements for upstream feedstock drying and carbonisation. In the resulting biochar, carbonisation accelerates the removal of polar functional groups and the rearranging of ring or linear shaped organic structures into polycondensation aromatic sheets. Carbonisation requires low oxygen levels to occur while limiting CO2 and NOx production [33].
The thermochemical pyrolysis process works well in the absence of oxygen at temperatures between 350 and 550 °C [34]. The pyrolysis mechanism can be divided into three groups based on three principles: slow pyrolysis, fast pyrolysis, and flash pyrolysis. Different pyrolysis systems have different operating conditions and outcomes. During slow pyrolysis, the heating rate is less than 1 °C/s, the pyrolysis temperature ranges between 300 °C and 700 °C, and the pyrolysis residence time exceeds 450 s. Fast pyrolysis occurs at temperatures between 500 °C and 1250 °C, with a heating rate is between 10 °C and 300 °C/s. Pyrolysis takes 0.5 to 200 s to complete. Lastly, the flash pyrolysis heating rate exceeds 1000 °C/s, and the pyrolysis temperature ranges between 800 °C and 1300 °C. Pyrolysis takes less than 0.5 s to complete [35].
Long polymeric chains of cellulose, lignin, hemicellulose, pectin, and other polymers make up the majority of biomass. During the pyrolysis process, larger molecules of organic materials begin to disintegrate into smaller molecules, which are discharged from the process stream as gases, condensable vapours, and solid char. Temperature, time, heating rate, and pressure, as well as the types of precursors and reactor design and configuration, all influence the proportion of each end product. In pyrolysis operations, the moisture content of biomass is also important. During the fast pyrolysis process, the moisture level of the feedstock should be around 10% [31].
Co-pyrolysis and normal pyrolysis have almost identical pathways. The procedure is essentially carried out in a closed reactor environment with a low operating temperature and no oxygen. The co-pyrolysis process has three basic steps that are necessary for oil production: sample preparation, co-pyrolysis, and condensation [36]. During pyrolysis, oxygen-containing compounds (aldehydes, phenols, ketones, and organic acids) are formed, making the bio-oil unstable and acidic. Catalytic pyrolysis can produce a high-quality bio-oil. As opposed to non-noble metal catalysts, a noble metal-supported catalyst can produce bio-oil from low oxygen-containing compounds. The produced bio-oil can be refined for use in petroleum refineries [37]. The pyrolysis reaction kinetics for lake sediment may be characterised using the Arrhenius equation in Equation (4).
dadt=k (T) f (a)=A exp (−ERT) f (a)(6)
where f (a) is the mechanism function equation, A is the preexponential factor (s−1), E is the activation energy (kJ/mol), and R is the universal gas constant (J/mol·K), a is the reaction conversion degree, t is the time (min) and T is the temperature (K). The value a is obtained by solving the following Equation (5).
a=wi−wwi−wf (7)
where w is the mass of the sample at time t (mg), and wi and wf are the sample’s initial and final masses (mg), respectively [38]. For energy computation, the energy flux into and out of the pyrolysis system is included in the control volume, as shown in Figure 2. The electrical energy for the reactor and the energy from the biomass are considered energy inputs. The energy outputs are the energy from bio-oil, charcoal, and non-condensable gases (NCG). The system boundary is defined under ambient conditions, as shown in Figure 2. As a result, heat transfer is no longer an energy contributor. Other energy sources and outputs are minor and insignificant [39].
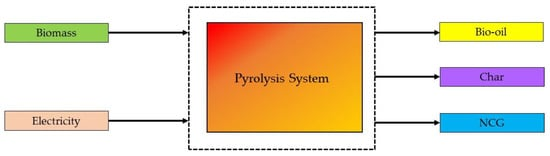
Figure 2. The schematic representation of energy flow process in a pyrolysis system [40].
The solid residue left over from the pyrolysis process may contain significant levels of heavy metals, necessitating the use of a stabilisation technique. The vitrification process has been investigated as a disposal solution that is close to the zero-landfilling scenario because it produces an invert vitreous matrix that can be used as a raw material in the glass industry or as an additive for cement, mortars, and plasters; additionally, it allows the simultaneous stabilisation of other materials with disposal problems [40].
2.3. Combustion
Combustion is a complex process involving mass transfer and fluid discharge as well as combined heat and chemical reactions [37]. The volume ratios of oxygen and nitrogen in binary mixtures of air are 21% and 79%, respectively. Further constituents may be found in an air mixture, but this is unimportant for combustion and gross analysis in this chemical reaction. The combustion process produces a hot gas with temperatures ranging from 700 to 1000 °C. For biomass combustion, the biomass feedstock must be pre-dyed at around 50% humidity [41]. As a result, combustion is a cost-effective technology for converting biofuel and producing heat. Biofuel production is not exactly modern technology, because no biofuels are generated until the complete combustion of the raw feedstock. Residual gas occurs in the complete or partial combustion of hydrocarbons in the case of oxygen, which includes a few combustion components and gaseous blends [42].
The ability of fuel molecular structures to reproduce the important radical species that affect endothermic and exothermic properties throughout the reaction history, radical consumption, regeneration, and chain branching descriptions for the relevant combustion behaviours are all important influences on combustion kinetic phenomena. The required behaviours for long hydrocarbons typically change with reaction temperature and pressure, resulting in pressure and temperature regimes governed by widely diverse reaction pathways [43]. At high temperature (>1100 K), fuel oxidative pyrolysis, fragment interactions, and the reactions of CO + OH ↔ CO2 + H and H + O2 ↔ O + OH are important mechanisms that determine the heat release rate and radical histories [44].
For well-maintained lean combustion systems, the assumption of full combustion, in which all of the fuel carbon reacts to carbon dioxide, all the fuel hydrogen responds to H2O, all of the fuel sulphur reacts to SO2, and all of the fuel nitrogen reacts to N2, is usually reasonable. This assumption is a good initial approximation for the species and energy balances. At high temperatures, other species such as CO and NO are present in considerable amounts in combustion products [45]. Food residue (40 to 50%), paper (30 to 40%), plastic (12%), fibre (3%), and other materials were often found in the separated municipal solid waste (MSW) following the mechanical treatment stage of the Korean mechanical biological treatment system. Season, culture, and other factors influence the composition of MSW (e.g., food residue and paper components can range between 70% and 80% of the total of MSW) [46].
2.4. Torrefaction
Torrefaction, a thermochemical technology, is thought to be an easy and inexpensive way to convert the properties of biomass to be almost identical to those of coal [47]. The Energy Centre of the Netherlands (ECN) was the first to investigate coal and torrefied biomass co-firing for energy generation, resulting in a comprehensive ECN study in 2005 [48]. An increase in torrefaction residence time resulted in a decrease in the hydrogen and oxygen composition of biomass in comparison to carbon, resulting in a decrease in the volatile matter content of biomass [49]. Torrefaction has also been widely identified as a promising thermal pre-treatment method for biochar processing, converting low-quality biomass into a high-energy-density, low-moisture feedstock [50].
Torrefaction is characterised by the partly regulated and isothermal pyrolysis of biomass at temperatures ranging from 200 to 300 °C. It is a method of slowly heating biomass in an inert environment to a maximum temperature of 300 °C. When compared to raw biomass, the treatment produces a solid, uniform product with lower moisture and higher energy content. Unbound water is removed from the biomass during the initial heating of torrefaction. The thermal condensation mechanism extracts the bound water at temperatures above 160 °C. The decomposition of hemicellulose begins when the temperature is increased from 180 to 270 °C. The process becomes fully exothermic at a temperature above 280 °C, resulting in a significant increase in the output of CO2, phenols, acetic acid, and other higher hydrocarbons [51].
Torrefaction is the process of devolatilisation and carbonisation of biomass polymers. All these polymers do not always degrade completely within the restricted temperature range of torrefaction between 200 and 300 °C. Different temperature ranges cause different polymers to deteriorate. Examples of qualitative values as given: hemicellulose (225 to 300 °C), cellulose (305 to 375 °C), and lignin (250 to 500 °C) [52]. Solid, liquid, and gaseous products are produced during the torrefaction process. The solid component is mostly char, with some sugar, polymeric structures, and ash thrown in the mix. CO, CO2, and small amounts of CH4 are among the non-condensable gases. Water from thermal decomposition, lipids such as terpenes and waxes, and organics such as alcohols and furans make up condensed liquid. Carbon water, carbon dioxide, carbon monoxide, acetic acid, methanol, and formic acid are all products of torrefaction. Decarboxylation is the process that produces CO2. Acetic acid is formed when the acetyl pendant group in cellulose decomposes. Carbon monoxide is produced primarily by the reaction of CO2 and steam with the porous char surface of biomass [53].
The kinetic energy of Miscanthus is greater than that of wheat straw, implying that the torrefaction of Miscanthus requires more energy than the torrefaction of wheat straw. Similarly, higher activation energy indicates a smooth reaction, whereas lower activation energy indicates a fast reaction. Torrefied biomass is more reactive than raw biomass [54]. Figure 3 indicates that about 70% of the original biomass weight and 90% of the original biomass energy are recovered, while the remainder (30% biomass weight and 10% biomass energy) is discharged as liquids and gases. As a result, it is viewed as a potential for studying wastes as a torrefaction feedstock [55].
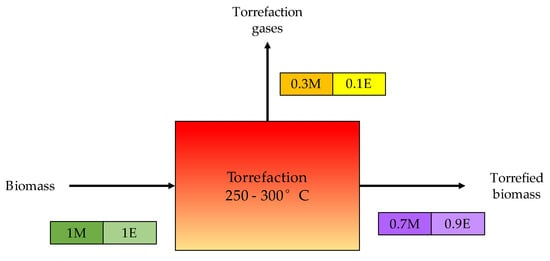
Figure 3. Typical mass and energy balance of the torrefaction process [55].
2.5. Hydrothermal Liquefaction
Hydrothermal liquefaction is a thermochemical process that transforms wet biomass into a liquid fuel [56]. Liquid bio crude oil is the main component of this reaction, with solid, liquid, and gaseous by-products. Liquefaction occurs in thermally compressed water at temperatures ranging from 250 to 550 °C and compressed pressures ranging from 5 to 25 MPa. There is no need for a drying phase or digestate method in this reaction [57]. Biocrude, a component of petroleum oil, is the primary commodity. Biocrude has a lot of potential now that has been strengthened. The use of alkaline catalysts reduces the deposition of char while increasing the yield and strength of the oil [58]. Hydration is a chemical reaction in which the principal chemicals in cement create chemical interactions with water molecules, resulting in the formation of hydrates. The next part delves into the hydration process in greater depth. To avoid side reactions that could weaken the concrete or interfere with the hydration process, the water must be pure. Water plays a vital role in the construction of “ideal” concrete, since the water-to-cement ratio is the most important aspect. Too much water will weaken the concrete, while too little will render it unusable.
Concrete must be workable to be solidified and moulded into various shapes. When creating concrete, a careful balance of the cement-to-water ratio is essential, since it must be both strong and workable [59]. Through-solution processes are also common in hydration processes involving acid–base interactions. Ca(OH)2 and glassy SiO2 do not react significantly when mixed as dry powders, but in the presence of liquid water, Ca(OH)2 dissolves significantly, and the resulting solution’s alkalinity is sufficient to promote silica hydrolysis; then, the reaction between calcium and silicate ions in alkaline solution is rapid, precipitating calcium silicate hydrate (C-S-H) gel. This emphasises the relevance of reactive species availability; their presence does not always imply that they are available for response. For example, most natural silicas and aluminosilicates are insoluble in water at close to neutral pH and so unreactive even when dissolved aqueous calcium or magnesium ions are present. However, as alkalinity rises, their solubility rises, resulting in higher aqueous silicate and aluminate concentrations and faster reaction rates. As a result, the solubility properties of the reactants in the aqueous phase have a significant impact on cement hydration reaction rates [60].
Among all of the conversion processes, hydrothermal carbonisation (HTC) is a promising technology. It is a thermochemical method that pre-heats high-moisture biomass with hot compressed water, making it useful for a variety of applications. It is carried out in a closed reactor at 180 to 280 °C and 2 to 6 MPa for 5 to 240 min [61]. Hydrothermal carbonisation generates a coal-like substance termed hydro char as its principal product as well as aqueous and gas phases as by-products [62]. The amount of hydro char produced depends on the process parameters and feedstock used. Decarboxylation, dehydration, and polymerisation are the major mechanisms involved in this process. Wet biomass makes a good solvent and reaction environment because its ionic product is greatest at temperatures between 200 and 280 °C, in which water may serve as both a base and an acid. Furthermore, the dielectric constant of water decreases at high temperatures, making it behave more as a nonpolar solvent. The benefit of hydrothermal carbonisation is that biomass can be converted to carbonaceous solids without the need for an energy-intensive drying process. The energy-to-weight ratio of hydro char is higher than that of the initial material [63].
2.6. Comparison of Thermal Technologies
Table 1 shows the comparison of five typical thermochemical processes. As can be seen, each of the given processes is subjected to a range of temperatures and pressures for the conversion and to obtain the desired results.
Table 1. Comparison of five thermochemical conversion processes.
| Thermochemical Process | Temperature (°C) | Pressure (MPa) | Gas Products | Pollutants | Purpose | Advantages |
|---|---|---|---|---|---|---|
| Gasification | 500 to 1300 | ≥0.1 | CO2, H2, CO2, H2O, and CH4 | H2S, NH3, tar, and dust | Converting biomass to high HV gas | Production of a wide range of chemical products and the ability to adapt to changing market conditions. |
| Pyrolysis | 300 to 1000 | 0.1 to 0.5 | CO, H2, CH4, and other hydrocarbons | H2S, NH3, tar, and dust | Converting biomass to biochar and bio-oil | Liquid fuels are produced directly, and after appropriate treatment, it can be directly treated in conventional refineries. |
| Combustion | 700 to 1000 | ≥0.1 | CO2 and H2O | SOxy, NOxy, polycyclic aromatic hydrocarbons (PAHs), and dust | Converting biomass to heat and electricity | The procedure is straightforward. Co-combustion of biomass and coal does not necessitate any changes to existing power plants. |
| Torrefaction | 200 to 300 | ≥0.1 | CO2, CO, and CH4 | H2S, COS, CS2, NH3, and HCN | Converting biomass into coal-like material | Moisture reduction, energy density increase, O/C ratio reduction, and improved ignitability and reactivity of the processed fuel. |
| Hydrothermal liquefaction | 250 to 550 | 5 to 25 | H2, CO, CO2, and CH4 | Polypropylene (PE), polypropene (PP), and nylon-6 (NY) | Converting wet biomass into crude-like oil | Process is environmentally friendly. The energy efficiency of the HTL process is very high. |
The most common thermochemical reactions are combustion, torrefaction, pyrolysis, liquefaction, and gasification. When comparing pyrolysis and gasification, the pyrolysis process has a lower reaction temperature than the gasification process. The material’s volatile components are thermally decomposed into more syngas and non-volatile carbon char, which are by-products of the pyrolysis process. The drawback of torrefaction is that as the residence time increases, the hydrogen and oxygen composition of biomass decreases in contrast to carbon, resulting in a decrease in biomass volatile matter content. The liquefaction method has received a lot of attention for utilising biomass waste because of its flexibility and potential to be used as a construction medium for a final product that incorporates all of the positive functional groups present in the liquefying solvents and biomass. Compared to other thermochemical processes, liquefaction requires lower temperatures, allowing it to save more fuel, generate fewer pollutants, and be considerably less expensive.
This entry is adapted from the peer-reviewed paper 10.3390/pr9091610
References
- Abokyi, E.; Appiah-Konadu, P.; Abokyi, F.; Oteng-Abayie, E.F. Industrial growth and emissions of CO2 in Ghana: The role of financial development and fossil fuel consumption. Energy Rep. 2019, 5, 1339–1353.
- Charles Rajesh Kumar, J.; Majid, M.A. Renewable energy for sustainable development in India: Current status, future prospects, challenges, employment, and investment opportunities. Energy Sustain. Soc. 2020, 10, 1–36.
- Chen, W.H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866.
- Sharma, A.; Jakhete, A.; Sharma, A.; Joshi, J.B.; Pareek, V. Lowering greenhouse gas (GHG) emissions: Techno-economic analysis of biomass conversion to biofuels and value-added chemicals. Greenh. Gases Sci. Technol. 2019, 9, 454–473.
- Pandey, B.; Prajapati, Y.K.; Sheth, P.N. Recent progress in thermochemical techniques to produce hydrogen gas from biomass: A state of the art review. Int. J. Hydrogen Energy 2019, 44, 25384–25415.
- Roman, K.; Barwicki, J.; Hryniewicz, M.; Szadkowska, D.; Szadkowski, J. Production of electricity and heat from biomass wastes using a converted aircraft turbine ai-20. Processes 2021, 9, 364.
- Ong, H.C.; Chen, W.H.; Singh, Y.; Gan, Y.Y.; Chen, C.Y.; Show, P.L. A state-of-the-art review on thermochemical conversion of biomass for biofuel production: A TG-FTIR approach. Energy Convers. Manag. 2020, 209, 112634.
- Bahng, M.K.; Mukarakate, C.; Robichaud, D.J.; Nimlos, M.R. Current technologies for analysis of biomass thermochemical processing: A review. Anal. Chim. Acta 2009, 651, 117–138.
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597.
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.W.; Bridgwater, A.V. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715.
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322.
- Niu, S.; Zhou, Y.; Yu, H.; Lu, C.; Han, K. Investigation on thermal degradation properties of oleic acid and its methyl and ethyl esters through TG-FTIR. Energy Convers. Manag. 2017, 149, 495–504.
- Arabi, M.; Ostovan, A.; Bagheri, A.R.; Guo, X.; Wang, L.; Li, J.; Wang, X.; Li, B.; Chen, L. Strategies of Molecular Imprinting-Based Solid-Phase Extraction Prior to Chromatographic Analysis; Elsevier B.V.: Amsterdam, The Netherlands, 2020.
- Naik, P. Analytical Techniques in Biochemistry; Springer: New York, NY, USA, 2016; p. 624.
- Chan, Y.H.; Cheah, K.W.; How, B.S.; Loy, A.C.M.; Shahbaz, M.; Singh, H.K.G.; Yusuf, N.R.; Shuhaili, A.F.A.; Yusup, S.; Ghani, W.A.W.A.K.; et al. An overview of biomass thermochemical conversion technologies in Malaysia. Sci. Total Environ. 2019, 680, 105–123.
- Abdulrazik, A.; Elsholkami, M.; Elkamel, A.; Simon, L. Multi-products productions from Malaysian oil palm empty fruit bunch (EFB): Analyzing economic potentials from the optimal biomass supply chain. J. Clean. Prod. 2017, 168, 131–148.
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 343–362.
- Katsaros, G.; Pandey, D.S.; Horvat, A.; Aranda Almansa, G.; Fryda, L.E.; Leahy, J.J.; Tassou, S.A. Experimental investigation of poultry litter gasification and co-gasification with beech wood in a bubbling fluidised bed reactor—Effect of equivalence ratio on process performance and tar evolution. Fuel 2020, 262, 116660.
- Phillips, D.; Mitchell, E.J.S.; Lea-Langton, A.R.; Parmar, K.R.; Jones, J.M.; Williams, A. The use of conservation biomass feedstocks as potential bioenergy resources in the United Kingdom. Bioresour. Technol. 2016, 212, 271–279.
- Boerrigter, H.; Rauch, R. Review of applications of gases from biomass gasification. ECN Biomass Coal Environ. 2006, 20, 33.
- Zhang, Y.; Zhao, Y.; Gao, X.; Li, B.; Huang, J. Energy and exergy analyses of syngas produced from rice husk gasification in an entrained flow reactor. J. Clean. Prod. 2015, 95, 273–280.
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109546.
- Ozturk, M.; Dincer, I. Integrated Gasification Combined Cycles; Comprehensive Energy System: Oshawa, ON, Canada, 2018; pp. 364–473.
- Kamble, A.D.; Saxena, V.K.; Chavan, P.D.; Mendhe, V.A. Co-gasification of coal and biomass an emerging clean energy technology: Status and prospects of development in Indian context. Int. J. Min. Sci. Technol. 2019, 29, 171–186.
- Guan, G.; Kaewpanha, M.; Hao, X.; Abudula, A. Catalytic steam reforming of biomass tar: Prospects and challenges. Renew. Sustain. Energy Rev. 2016, 58, 450–461.
- Ren, J.; Cao, J.P.; Yang, F.L.; Zhao, X.Y.; Tang, W.; Cui, X.; Chen, Q.; Wei, X.Y. Layered uniformly delocalized electronic structure of carbon supported Ni catalyst for catalytic reforming of toluene and biomass tar. Energy Convers. Manag. 2019, 183, 182–192.
- Liu, N.A.; Fan, W.; Dobashi, R.; Huang, L. Kinetic modeling of thermal decomposition of natural cellulosic materials in air atmosphere. J. Anal. Appl. Pyrolysis 2002, 63, 303–325.
- Xu, C.; Hu, S.; Xiang, J.; Zhang, L.; Sun, L.; Shuai, C.; Chen, Q.; He, L.; Edreis, E.M.A. Interaction and kinetic analysis for coal and biomass co-gasification by TG-FTIR. Bioresour. Technol. 2014, 154, 313–321.
- Seo, Y.-C.; Alam, M.T.; Yang, W.-S. Gasification of Municipal Solid Waste. Gasif. Low Grade Feed. 2018.
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Thermodynamics of gas-char reactions: First and second law analysis. Chem. Eng. Sci. 2003, 58, 1003–1011.
- Ivanova, N.; Gugleva, V.; Dobreva, M.; Pehlivanov, I.; Stefanov, S.; Andonova, V. We are IntechOpen, the World’s Leading Publisher of Open Access Books Built by Scientists, for Scientists TOP 1%; IntechOpen: London, UK, 2016.
- Collard, F.X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608.
- Novak, J.M.; Johnson, M.G. Elemental and Spectroscopic Characterization of Low-Temperature (350 °C) Lignocellulosic-and Manure-based Designer Biochars and Their Use as Soil Amendments; Elsevier Inc.: Amsterdam, The Netherlands, 2018.
- Sebastiani, A.; Macrì, D.; Gallucci, K.; Materazzi, M. Steam—oxygen gasification of refuse derived fuel in fluidized beds: Modelling and pilot plant testing. Fuel Process. Technol. 2021, 216, 106783.
- Zhang, Y.; Chen, P.; Liu, S.; Peng, P.; Min, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Fan, L.; Liu, C.; et al. Effects of feedstock characteristics on microwave-assisted pyrolysis—A review. Bioresour. Technol. 2017, 230, 143–151.
- Abnisa, F.; Wan Daud, W.M.A. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85.
- Bhoi, P.R.; Ouedraogo, A.S.; Soloiu, V.; Quirino, R. Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew. Sustain. Energy Rev. 2020, 121, 109676.
- Wu, Z.; Luo, H. Pyrolysis Characteristics and Kinetic Analysis of Sediment from the Dianchi Lake in China. Int. J. Chem. Eng. 2018, 2018.
- Boateng, A.A.; Mullen, C.A.; Osgood-Jacobs, L.; Carlson, P.; Macken, N. Mass Balance, Energy, and Exergy Analysis of Bio-Oil Production by Fast Pyrolysis. J. Energy Resour. Technol. 2012, 134, 042001.
- Grigiante, M.; Ischia, M.; Baratieri, M.; Maschio, R.D.; Ragazzi, M. Pyrolysis analysis and solid residue stabilization of polymers, waste tyres, spruce sawdust and sewage sludge. Waste Biomass Valorization 2010, 1, 381–393.
- Salimbeni, A. Techno-Economic Assessment of Lignocellulosic Biomass Energy Conversion by Slow Oxidative Pyrolysis. Master’s Thesis, University of Florence, Florence, Italy, 2016.
- Zhang, Y.; Cui, Y.; Chen, P.; Liu, S.; Zhou, N.; Ding, K.; Fan, L.; Peng, P.; Min, M.; Cheng, Y.; et al. Gasification Technologies and Their Energy Potentials; Elsevier B.V.: Amsterdam, The Netherlands, 2019.
- Dooley, S.; Won, S.H.; Dryer, F.L. Surrogate Fuels and Combustion Characteristics of Liquid Transportation Fuels; Elsevier B.V.: Amsterdam, The Netherlands, 2019.
- Westbrook, C.K. Chemical kinetics of hydrocarbon ignition in practical combustion systems. Proc. Combust. Inst. 2000, 28, 1563–1577.
- Caretto, L. Introduction to Combustion Today′s Class; Spring: Long Beach, CA, USA, 2010; pp. 1–22.
- Kim, D.; Yoshikawa, K.; Lee, K.; Park, K.Y. Investigation of the combustion characteristics of municipal solid wastes and their hydrothermally treated products via thermogravimetric analysis. J. Mater. Cycles Waste Manag. 2015, 17, 258–265.
- Ribeiro, J.M.C.; Godina, R.; Matias, J.C.D.O.; Nunes, L.J.R. Future perspectives of biomass torrefaction: Review of the current state-of-the-art and research development. Sustainability 2018, 10, 2323.
- Bergman, P.C.A.; Boersma, A.R.; Zwart, R.W.R.; Kiel, J.H. Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations (BIOCOAL); C-05-013; ECN: Petten, The Netherlands, 2005; pp. 1–72.
- Bach, Q.V.; Skreiberg, O. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677.
- Yue, Y.; Singh, H.; Singh, B.; Mani, S. Torrefaction of sorghum biomass to improve fuel properties. Bioresour. Technol. 2017, 232, 372–379.
- Tumuluru, J.S.; Sokhansanj, S.; Wright, C.T.; Kremer, T. GC Analysis of Volatiles and Other Products from Biomass Torrefaction Process; IntechOpen: London, UK, 2012.
- Basu, P. Chp. 04: Torrefaction; Biomass Gasification, Pyrolysis and Torrefaction: Halifax, NS, Canada, 2013; pp. 93–154. ISBN 9780128129920.
- White, R.H.; Dietenberger, M.A. Wood Products: Thermal Degradation and Fire. Encycl. Mater. Sci. Technol. 2001, 9712–9716.
- Acharya, B.; Pradhan, R.R.; Dutta, A. Qualitative and kinetic analysis of torrefaction of lignocellulosic biomass using DSC-TGA-FTIR. AIMS Energy 2015, 3, 760–773.
- Karki, S.; Poudel, J.; Oh, S.C. Thermal pre-treatment of sewage sludge in a lab-scale fluidized bed for enhancing its solid fuel properties. Appl. Sci. 2018, 8, 183.
- Minowa, T.; Kondo, T.; Sudirjo, S.T. Thermochemical liquefaction of Indonesian biomass residues. Biomass Bioenergy 1998, 14, 517–524.
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624.
- Zhou, Y.; Hu, C. Catalytic thermochemical conversion of algae and upgrading of algal oil for the production of high-grade liquid fuel: A review. Catalysts 2020, 10, 145.
- Lv, S.H. High-performance superplasticizer based on chitosan. Biopolym. Biotech. Admix. Eco Effic. Constr. Mater. 2016, 131–150.
- Gartner, E.M.; MacPhee, D.E. A physico-chemical basis for novel cementitious binders. Cem. Concr. Res. 2011, 41, 736–749.
- Weiner, B.; Baskyr, I.; Poerschmann, J.; Kopinke, F.D. Potential of the hydrothermal carbonization process for the degradation of organic pollutants. Chemosphere 2013, 92, 674–680.
- Yoganandham, S.T.; Sathyamoorthy, G.; Renuka, R.R. Emerging Extraction Techniques: Hydrothermal Processing; Elsevier Inc.: Amsterdam, The Netherlands, 2020.
- Ischia, G.; Fiori, L. Hydrothermal Carbonization of Organic Waste and Biomass: A Review on Process, Reactor, and Plant Modeling. Waste Biomass Valorization 2021, 12, 2797–2824.
