Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
Phenolic acids comprise a class of phytochemical compounds that can be extracted from various plant sources and are well known for their antioxidant and anti-inflammatory properties.
- phenolic acids
- antioxidants
- health properties
1. Introduction
Free radicals are mainly reactive oxygen species (ROS) (including hydroxyl-, superoxide-radicals and singlet oxygen) that are formed in tissue cells by various endogenous and exogenous pathways. ROS normally exert an adverse impact on human health by inducing the so called “oxidative stress conditions” [1]. The ability of free radicals to structurally modify cellular components and cause oxidative damage to biomolecules (LDL-low density lipoproteins, DNA, etc.) has revealed their involvement in a variety of health pathologies (i.e., inflammation, aging, types of cancer and cardiovascular diseases) [2,3].
Nature has generously offered several types of natural dietary antioxidants, among which phenolic compounds can operate as scavengers of free radicals in vivo and can efficiently reduce the harmful health impacts of oxidative damage [4,5]. Phenolic acids comprise a group of natural phenolic compounds that are present in a wide range of herbs and other species of the plant kingdom [6]. More specifically, thyme, oregano, rosemary, sage, and mint herbal preparations—all rich in various phenolics—have been reported to exert strong antioxidant biochemical and anti-inflammatory properties [7,8]. A few authors have reviewed the radical scavenging capacity of phenolic acids and their subsequent beneficial effects against the development of cancer, cardiovascular diseases and other health disorders (such as skin problems, inflammations, bacterial infections, etc.) [9]. The main biochemical pathways and mechanisms of phenolic actions against the development of certain types of cancer include: free radical scavenging, enzyme induction, DNA damage repair, cell proliferation depression, and apoptosis [10].
2. Structural Classification of Natural Phenolic Acids
In terms of their chemical structure, phenolic acids are classified as:
Hydroxybenzoic acids with a C6-C1 structure: Among them a trihydroxy derivative (gallic acid) has been associated with tea antioxidant activity, while vanilic acid is a methoxy-hydroxy derivative serving as a well-known flavouring agent [12].
Hydroxycinnamic acids with a C6-C3 structure[13]: These are abundant in plant sources, with p-coumaric (4-hydroxy derivative), caffeic (3, 4-dihydroxy derivative) and ferulic (3-methoxy, 4-hydroxy derivative) commonly present in various culinary herbs. In addition, rosmarinic acid (an ester of caffeic acid with 3,4-dihydroxyphenyl lactic acid) is mainly encountered in certain aromatic herbs [14].
Phenylacetic acids with a C6-C2 structure. Phenylacetic acids are scarce in fruits and vegetables, while a dihydroxy derivative was detected in strawberry tree honey [15]. Carnosic acid belongs to the phenolic diterpenes that are usually classified as hybrid phenolics [13].
This review focuses on the most common hydroxybenzoic and hydroxycinnamic phenolic acids, along with carnosic acid, the chemical structures of which are given in Figure 1.
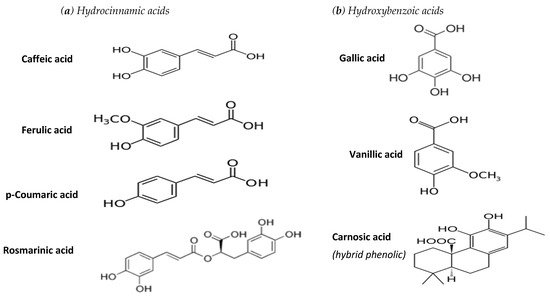
Figure 1. Chemical structure of phenolic acids examined in this study.
3. Herbal Sources and Extraction of Phenolic Acids
Caffeic acid (CA) is found at high levels in various herbs worldwide, including the South American herb yerba mate (1.5 g/kg) [16], the Japanese herbal leaf tea, the tea stem from Moringa oleifera L. [17], and thyme (1.7 mg/kg) [18].
Carnosic acid (CarA) can be found in a few species of the Lamiaceae family (such as rosemary and common salvia species). It has been reported to be present at a concentration of 1.5 to 2.5% in dried sage leaves [19,20].
Ferulic acid (FA) is present in black beans at an average concentration of 0.8 g/kg, while flaxseed has been reported as the richest natural source of FA glucoside (4.1 ± 0.2 g/kg), [21,22]. FA has been also identified as the major phenolic acid in Angelica sinensis (Oliv.), a traditional medicinal and edible plant in China [23].
Gallic acid (GA) has been found to be the main phenolic acid in tea [24] but also reported in high amounts in the parasitic plant Cynomorium coccineum, the aquatic plant Myriophyllum spicatum, and the blue-green alga Microcystis aeruginosa [25]. In addition, GA was recently identified as the main phenolic compound in leaf extracts from the medicinal halophyte Thespesia populnea tea [26].
p-Coumaric acid (p-CA) has been identified in basil, garlic [27] and in amaranth leaves and stem at a concentration range of 28–44 mg/kg [28]. p-CA has been reported as the major active compound in Bambusae Caulis, a Chinese medicinal herb [29] as well as in cultivars of husked oat (Avena sativa L.) in Finland [30].
Rosmarinic acid (RA) is the main phenolic component in several members of the Lamiaceae family, including among others: Rosmarinus officinalis, Origanum spp., Perilla spp., and Salvia officinialis in concentrations varying between 0.05 and 26 g/kg dry weight [31]. Additionally, the results of Tsimogiannis et al. [32] indicate an amount of 19.5 g/kg in the leaves of pink savoury (Satureja thymbra L.).
Vanillic Acid (VA) is commonly found in several fruits, olives, and cereal grains (e.g., whole wheat), as well as in wine. VA was also identified in fruit extracts of the açaí palm plant (Euterpe oleracea) [33] and in the root of Angelica sinensis (an herb indigenous to China) at concentrations between 1.1 and 1.3 g/kg [34].
4. Extraction of Phenolic Acids from Their Natural Sources
The extraction and identification of phenolic acids has been studied by various researchers [35,36]. Phenolic acids are compounds with medium to high polarity and, therefore, can be extracted by water [37]. Nevertheless, aqueous solutions of ethanol or acetone (50–70%) are the best solvents for the quantitative extraction of hydrocinnamic acids [38]. On the contrary, CarA exhibits low polarity and is quantitatively extracted with the use of pure acetone or ethanol [39].
Hydroxycinnamic and hydroxybenzoic acids may be linked to polysaccharides of the cell walls by ester bonds and to lignin components by ester or ether bonds [40]. Mild alkaline hydrolysis can be implied to cleave the ester bonds, while acid hydrolysis to cleave the ether bonds and release the phenolic acids [41]. However, phenolic acids may be degraded under alkaline conditions, e.g., RA has been reported to transform to CA [42]. Additionally, mild temperature and time combinations are suggested to avoid degradation. The most prone to degradation is CarA, which is oxidised to carnosol (which also exhibits antioxidant activity) at temperatures higher than 50 °C and at longer extraction times [43].
In addition to conventional solid liquid extraction, ultrasound assisted extraction and microwave assisted extraction proved even more effective for phenolic acid extraction, while shortening extraction time [44,45].
The predominant role of high-performance liquid chromatography (HPLC) in the definition of the phenolic profile of various plant sources has been recently examined by Ciulu et al. (2018) [46], who also present the most recently developed mass spectrometry-based detection systems. In addition, the various developed procedures for the quantification of phenolic compounds have been described in the literature, along with the spectrophotometric protocols for the evaluation of their antioxidant properties [47,48].
This entry is adapted from the peer-reviewed paper 10.3390/molecules26175405
This entry is offline, you can click here to edit this entry!
