The total antioxidant capacity of seminal plasma is a very common way of presenting the antioxidant capabilities of the seminal plasma. This term is used to evaluate the total ability of a fluid to scavenge free radicals in solution. In general, fertile men have seminal plasma with a higher total antioxidant capacity than that of infertile patients.
- antioxidants
- fertility couple
- reactive oxygen species
- seminal plasma
1. Introduction
As a reproductive health disease, infertility is defined as an incapacity to generate and maintain a full-term pregnancy after a year of regular sexual intercourse without the use of any contraceptive method [1]. According to the World Health Organization (WHO), around 9% of the couples worldwide present fertility issues [2]. Females or males may have infertility problems. For many years, infertility issues were mainly attributed to women, but nowadays, such problems are attributed to both sexes in almost equal parts [2]. Male and female reproductive tract fluids have a crucial role on fertility. Indeed, the fertility of the couple is dependent on the chemical composition of these fluids. Seminal plasma in men and vaginal, uterine, and oviductal fluid in the female reproductive tract allow the nourishment of gametes and assist their fertilization. Among all the molecules that compose the reproductive fluids, those with antioxidant capabilities have been gaining interest. Antioxidants levels in reproductive fluids have been positively correlated to human fertility, independent of sex.
In this review, we will discuss the composition of antioxidants in the seminal plasma and female reproductive tract fluids and its relationship with the fertility of individuals. Moreover, the most recent information on the role of the reproductive fluids and their antioxidant profile on the processes that allow fertilization and embryo development will be discussed.
2. Constitution and Functions of Seminal Plasma
Semen constitutes a mixture of cells and liquids in a mixture of 5 to 95%, respectively [3]. The cellular fraction of this fluid constitutes the spermatozoa, epithelial cells, and leucocytes, while the non-cellular liquid fraction of semen is known as seminal plasma. Seminal plasma is a very complex and important fluid in the function of the ejaculate. It is formed by secretions from the rete testis, epididymis, and accessory glands [4][5]. The contribution of each component suffers slight variations: 2–5% are secretions from the testis, 20–30% from the prostate, approximately 1% from bulbourethral and periurethral glands, and the major contribution is 65–75% from the seminal vesicles ( Figure 1 ) [3]. Fructose and other sugars, amino acids, peptides and proteins, lipids, cytokines, microRNA, and inorganic ions are its main constituents.
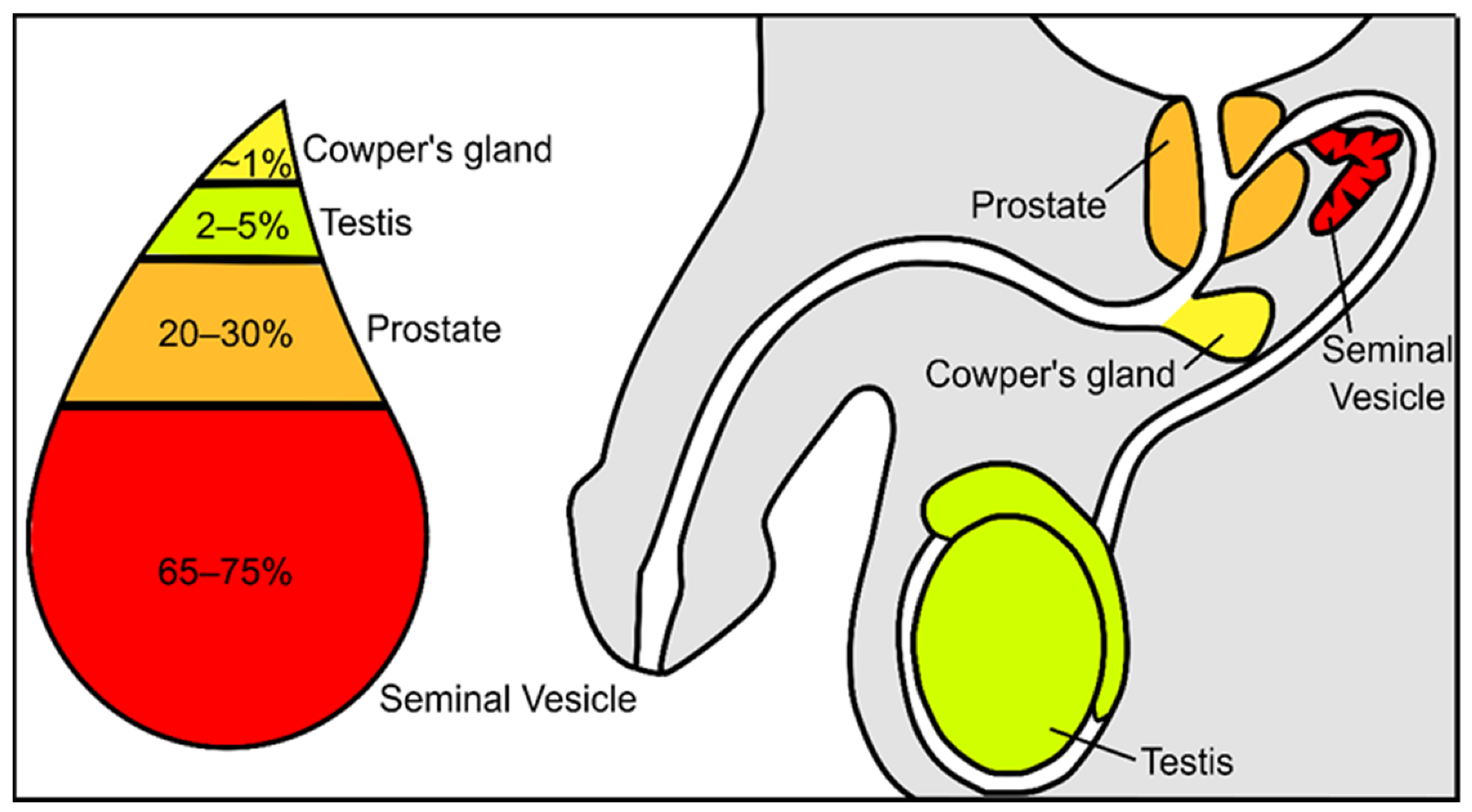 Figure 1. Schematic representation of the contribution from the different urogenital glands to the seminal plasma composition.
Figure 1. Schematic representation of the contribution from the different urogenital glands to the seminal plasma composition.
At first look, the role of seminal plasma seems to be related to the survival and transport of spermatozoa; however, this has been devaluated by the assisted reproductive technologies [5]. In fact, washed spermatozoa produce viable embryos. On the other hand, a lower fertility is observed when seminal plasma is diluted or removed in livestock species [6]. Although contradictory information can be found in the literature, is not possible to deny that seminal plasma must have a role in sperm function and in events in the male and female reproductive tracts, such as fertilization and embryo development [7]. Thus, it is worth studying the seminal plasma of men with fertility problems. The measurement of zinc, fructose, and neutral α-glucosidase content in seminal plasma are optional assays suggested by the WHO laboratory manual for the examination and processing of human semen [8]. Zinc has a protective effect on spermatozoa from bacteria and is important in testicular development and spermatogenesis [9][10][11]. In seminal plasma, fructose is mainly produced by the seminal vesicles [12] and has a role in spermatozoa metabolism [13] as a major energy source [14] for motility [13]. The determination of seminal fructose is useful in cases of inflammation of accessory glands and in cases of obstructive azoospermia. The content of fructose is low or undetectable when ejaculatory ducts are obstructed [15] or a congenital vas deferens-seminal vesicle developmental defect is present [16][17]. The determination of fructose concentration, as auxiliary diagnosis, is also useful in cases of retrograde ejaculation, when an abnormal concentration of this sugar is detected in urine after ejaculation [15]. The α-glucosidase is a marker for the epididymal function [18][19]; however, the measurement of α-glucosidase is not widely implemented and used [20]. Alterations in α-glucosidase are associated with a defective process of spermatozoa maturation in epididymis or inflammation in this structure [21][22].
The roles of seminal plasma, from the epididymis to fertilization, were studied in several species, although the available data highlights contradictory conclusions. Seminal plasma functions include: effects on sperm motility, mostly related to proteins found in this fluid [23][24][25]; providing nutrients and osmotic homeostasis for spermatozoa survival [26][27][28][29]; effects on the capacitation process and also on the avoidance of premature activation of capacitation [30][31][32]; an active role in the inflammatory response by the female reproductive tract [33][34][35]; the modulation of sperm-egg interactions; and effects on fertilization [36][37][38]. The importance of seminal fluid in fertilization events was investigated in studies where seminal plasma components were added to thawed sperm. It was reported that seminal plasma acts as a defense mechanism for spermatozoa from cells from the immune system [39]. Supplementation of cryopreservation medium with seminal plasma was proved to improve motility, membrane integrity, and in vitro fertilization (IVF) outcomes [40]. Similarly, it was described that seminal plasma added to post-thawed sperm medium has beneficial effects on sperm motility and membrane integrity [41][42]. This could be due to the known antioxidant capabilities of seminal plasma, which protects the sperm cells from oxidative damage. The antioxidant capabilities of seminal plasma are due to multiple nutrients of different sources on the male reproductive tract ( Table 1 ). These help in the control of excessive reactive oxygen species (ROS) production. Dysregulation of antioxidant secretion can lead to oxidative stress in the semen, which has been regarded as one of the main causes of male infertility in recent decades [43].
| Reproductive Glands | Antioxidants | References |
|---|---|---|
| Epididymis | Carnitines | [44] |
| Seminal Vesicle | Vitamin C | [44] |
| Prostate | Zinc | [45][46][47][48] |
| Selenium | ||
| Glutathione peroxidase | ||
| Glutathione reductase | ||
| Superoxide dismutase | ||
| Catalase | ||
| Albumin |
3. Seminal Plasma Antioxidant Capabilities and Relation to Male Fertility Outcomes
Another question worth asking is which antioxidants are of greater importance and which are found to be less represented in males with impaired fertility. As discussed, seminal plasma is a complex fluid, and in its composition are present multiple antioxidants that, herein, will be divided into protein and non-protein. In the next topics, it will be discussed the importance of the abundancy of non-protein antioxidants and the expression and activity of protein antioxidants on seminal plasma for male fertility outcomes (summarized in Table 1 ).
All the studies described above show that the lack of one specific non-protein antioxidant increases the ROS content of the seminal plasma. However, the information available highlights that the general lack of antioxidants is the cause of the enhanced oxidative reductive potential, and not one specific antioxidant. The lack of sufficient antioxidant capacity of the seminal plasma can lead to an increase in oxidative damage of sperm cells, seen in the form of DNA fragmentation, lipid and protein oxidation, and decreased mobility, which ultimately led to sperm cell apoptosis and diminished motility. Although some non-protein antioxidants seem to have more antioxidant relevance (e.g., vitamin C) than others, we could not find any study that showed a compensation of other non-protein antioxidant to restore the seminal plasma antioxidant capability. This is probably because the concentrations of most of these non-protein antioxidants in the organism is diet-dependent. Thus, the organism cannot simply produce more non-protein antioxidants. However, this is not the case for protein antioxidants, whose expression and activity can be regulated by the organism. The expression and activity of antioxidant proteins in individuals with subfertility/infertility will be discussed in more depth in the next topic.
In contrast to non-protein antioxidants, which are no longer effective after ROS neutralization [49], catalytic antioxidants are a renewable resource. The large majority of these enzymes are secreted into the seminal plasma by the epididymis and prostate [50].
The complex network of ROS management in a complex fluid such as the seminal plasma is a mechanism that still needs attention from the scientific community. The altered antioxidant protein expression and its relationship with the concentration of the different non-protein antioxidants and the contribution of each type of antioxidants to the total antioxidant capacity of the seminal plasma still have many secrets to be revealed.
4. Role of Seminal Plasma on Fertility Techniques and Importance of Its Antioxidants in the Female Reproductive Tract
The impact of the usage of seminal plasma on artificial reproductive techniques are starting to be studied in multiple species, including humans. For example, in cattle, the infusion of seminal plasma into the reproductive tracts of Holstein cattle increased the birth weight of heifer calves born after insemination with X-sorted semen but did not alter pregnancy or live birth statistics after insemination [51]. Seminal plasma also has an important role in the conception of embryos by IVF at the time of transfer into the female tract. In mice, embryo transfer protocols usually employ recipients exposed to seminal plasma by mating with vasectomized males. Fetal loss and other issues are greater when embryos are transferred after pseudopregnancy achieved with no exposure to seminal plasma [52]. In humans, a randomized trial in couples undergoing ART techniques has shown a significant improvement in embryo transfer after exposure to seminal plasma [53]. Hart and Norman demonstrated a higher proportion of transferred embryos in 6–8 weeks in women that were exposed to semen around the time of embryo transfer compared to those who abstained [54]. Some meta-analyses provided additional information about seminal plasma in clinical pregnancy rates. The meta-analysis conducted by Crawford and collaborators [55] demonstrated a significant improvement in clinical pregnancy rates, approximately 23%, while Saccone and collaborators demonstrate a 20% improvement [56]. However, other authors’ analysis concluded that seminal plasma may effectively increase clinical pregnancy rates, but the finding should be regarded with caution since there is not enough evidence to determine the impact on live births [57]. These data demonstrate that further studies are warranted to better quantify the impact of seminal plasma on live birth after ART techniques and thus find the difference between coitus and exogenous delivery of seminal plasma.
In human beings, the direct effect of seminal plasma signaling activity on long-term health is not clear. There are clinical observations that demonstrated the possible effects of seminal plasma in the female reproductive system, including in specific diseases. The impact of seminal plasma factors on human immunodeficiency viruses (HIV) infections in vivo has been investigated [58]. Sperm can assist in the efficient transmission of HIV [59] through the interaction of HIV and sperm heparin sulfate. However, other factors seemed to inhibit HIV infectivity; Mucin-6 and seminal plasma cationic peptides are responsible for this [60].
Despite all these immune suppressors, some excessive ROS formation is unavoidable. Some originate in the female medium (mainly by leukocytes in the cervix and vaginal microbiome) and others are believed to be formed by the sperm cells through their long journey to the oocyte [61]. The management of the latter was already discussed above. However, excessive female ROS formation could be an additional challenge from which the seminal plasma must protect the sperm cells. One example of an oxidative molecule present in the female reproductive tract that was found to have deleterious effect on sperm is the NO. Seminal plasma NO concentration is positively correlated to male infertility [62]. This particular ROS is important for the contraction of the uterine and oviduct cavities that also assist in sperm motility for oocyte fertilization [63]. However, this NO can also affect sperm. Interestingly, seminal plasma has an inhibitory role on the protein NO synthase activity in brain tissue [64]. One could assume that a similar process can be happening in the female reproductive tract for sperm protection against oxidative damage; however, this is only a hypothesis and tests should be made to confirm it.
Some examples of the importance of the reproductive fluid antioxidants to human fertility are described above and summarized at Figure 2 ; however, many other mechanisms are surely yet to be revealed. The study of the interactions between seminal plasma and the female reproductive fluid antioxidants still presents many obstacles, since it is challenging to study this process in vivo and almost impossible to replicate the condition in in vitro experiments. Despite not being able to fully understand yet the specific contribution that a good spermatozoon has on a damaged female reproductive tract through ROS or vice versa, it is undeniable that seminal plasma has multiple positive aspects in female reproductive stress to ensure proper fertilization and hence embryo development. Thus, understanding the modification of the female genital profile triggered by seminal components that interact with the internal genital lining during the ovulatory period may contribute to improving success rates and outcomes in fertility.
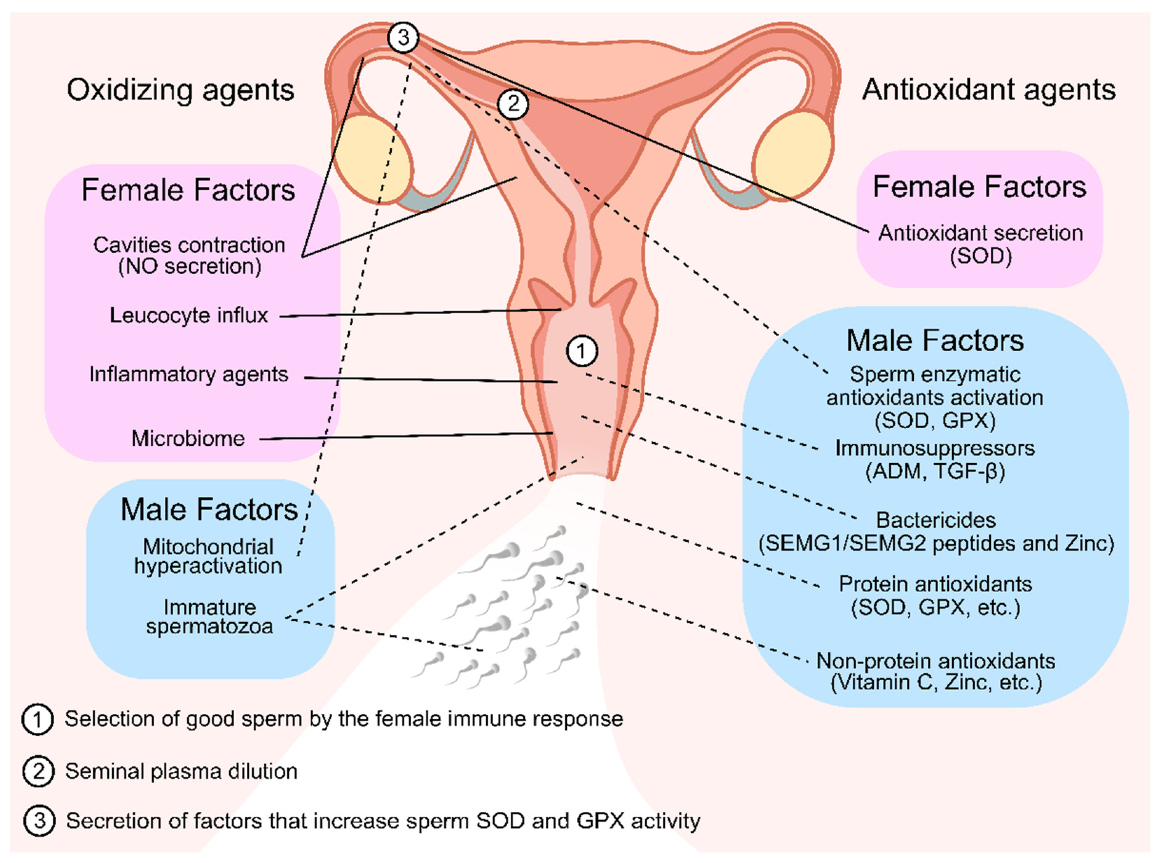 Figure 2. Oxidizing and antioxidant agents of seminal plasma and female reproductive tract fluid that are linked with selecting or protecting the sperm cells throughout their journey to fertilization. In the first stages of the sperm journey, they encounter multiple oxidizing agents in the vaginal fluid. To counter that, many antioxidizing agents are present in the seminal plasma. Both these facts contributed for the selection and protection of good sperm for fertilization (1). In later stages, after seminal plasma dilution (2), seminal plasma antioxidant protection is scarce; thus, it is possible that the oviductal epithelium secretes a factor that stimulates the sperm antioxidant defenses by increasing SOD and GPX activity (3) as well and secreting antioxidant enzymes to the lumen, which are necessary to protect the sperm from the endogenous ROS formation in the hyperactivation stage. Solid lines represent female factors. Dashed lines represent male factors. NO—Nitric Oxide; SOD—Superoxide Dismutase; GPX—Glutathione Peroxidase; SEMG—Semenogelins
Figure 2. Oxidizing and antioxidant agents of seminal plasma and female reproductive tract fluid that are linked with selecting or protecting the sperm cells throughout their journey to fertilization. In the first stages of the sperm journey, they encounter multiple oxidizing agents in the vaginal fluid. To counter that, many antioxidizing agents are present in the seminal plasma. Both these facts contributed for the selection and protection of good sperm for fertilization (1). In later stages, after seminal plasma dilution (2), seminal plasma antioxidant protection is scarce; thus, it is possible that the oviductal epithelium secretes a factor that stimulates the sperm antioxidant defenses by increasing SOD and GPX activity (3) as well and secreting antioxidant enzymes to the lumen, which are necessary to protect the sperm from the endogenous ROS formation in the hyperactivation stage. Solid lines represent female factors. Dashed lines represent male factors. NO—Nitric Oxide; SOD—Superoxide Dismutase; GPX—Glutathione Peroxidase; SEMG—Semenogelins
This entry is adapted from the peer-reviewed paper 10.3390/antiox10091441
References
- Larsen, U. Research on infertility: Which definition should we use? Fertil. Steril. 2005, 83, 846–852.
- Fainberg, J.; Kashanian, J.A. Recent advances in understanding and managing male infertility. F1000Research 2019, 8.
- Samanta, L.; Parida, R.; Dias, T.R.; Agarwal, A. The enigmatic seminal plasma: A proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 2018, 16, 41.
- Lutwak-Mann, T.M.a.C. Male Reproductive Function and Semen. Andrologia 1982, 14, 76.
- Juyena, N.S.; Stelletta, C. Seminal plasma: An essential attribute to spermatozoa. J. Androl. 2012, 33, 536–551.
- Tummaruk, P.; Lundeheim, N.; Einarsson, S.; Dalin, A.M. Reproductive Performance of Purebred Swedish Landrace and Swedish Yorkshire Sows: II. Effect of Mating Type, Weaning-to-first-service Interval and Lactation Length. Acta Agric. Scand. Sect. A—Anim. Sci. 2000, 50, 217–224.
- Bedford, J.M. The functions--or not--of seminal plasma? Biol. Reprod. 2015, 92, 18.
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen; WHO: Geneva, Switzerland, 2010.
- Madding, C.I.; Jacob, M.; Ramsay, V.P.; Sokol, R.Z. Serum and semen zinc levels in normozoospermic and oligozoospermic men. Ann. Nutr. Metab. 1986, 30, 213–218.
- Khan, M.S.; Zaman, S.; Sajjad, M.; Shoaib, M.; Gilani, G. Assessment of the level of trace element zinc in seminal plasma of males and evaluation of its role in male infertility. Int. J. Appl. Basic Med. Res. 2011, 1, 93–96.
- Lin, Y.; Chang, T.; Tseng, Y.; Lin, Y.; Huang, F.; Kung, F.; Chang, S. Seminal plasma zinc levels and sperm motion characteristics in infertile samples. Chang. Gung Med. J. 2000, 23, 260–266.
- Schoenfeld, C.; Amelar, R.D.; Dubin, L.; Numeroff, M. Prolactin, Fructose, and Zinc Levels Found in Human Seminal Plasma. Fertil. Steril. 1979, 32, 206–208.
- Videla, E.; Blanco, A.M.; Galli, M.E.; Fernández-Collazo, E. Human seminal biochemistry: Fructose, ascorbic acid, citric acid, acid phosphatase and their relationship with sperm count. Andrologia 1981, 13, 212–214.
- Trang, N.; Sang, T.; Hoang, N.; Khanh, N.; Duc, T. Assessment of the level of seminal zinc and fructose concentration in seminal plasma of Vietnamese infertile men. MOJ Biorg. Org. Chem. 2018, 2, 185–190.
- Lu, J.C.; Chen, F.; Xu, H.R.; Huang, Y.F.; Lu, N.Q. Standardization and quality control for determination of fructose in seminal plasma. J. Androl. 2007, 28, 207–213.
- Kise, H.; Nishioka, J.; Satoh, K.; Okuno, T.; Kawamura, J.; Suzuki, K. Measurement of protein C inhibitor in seminal plasma is useful for detecting agenesis of seminal vesicles or the vas deferens. J. Androl. 2000, 21, 207–212.
- Kumar, R.; Thulkar, S.; Kumar, V.; Jagannathan, N.; Gupta, N.P. Contribution of investigations to the diagnosis of bilateral vas aplasia. ANZ J. Surg. 2005, 75, 807–809.
- Cooper, T.G.; Yeung, C.H.; Nashan, D.; Nieschlag, E. Epididymal markers in human infertility. J. Androl. 1988, 9, 91–101.
- García Díez, L.C.; Esteban Ruiz, P.F.; Villar, E.; Corrales Hernandez, J.J.; Burgo, R.; Delgado, M.; Miralles, J.M. Enzyme and hormonal markers in the differential diagnosis of human azoospermia. Arch. Androl. 1992, 28, 181–194.
- Mahmoud, A.M.; Geslevich, J.; Kint, J.; Depuydt, C.; Huysse, L.; Zalata, A.; Comhaire, F.H. Seminal plasma alpha-glucosidase activity and male infertility. Hum. Reprod. 1998, 13, 591–595.
- Haidl, G.; Badura, B.; Hinsch, K.D.; Ghyczy, M.; Gareiss, J.; Schill, W.B. Disturbances of sperm flagella due to failure of epididymal maturation and their possible relationship to phospholipids. Hum. Reprod. 1993, 8, 1070–1073.
- Von der Kammer, H.; Scheit, K.H.; Weidner, W.; Cooper, T.G. The evaluation of markers of prostatic function. Urol. Res. 1991, 19, 343–347.
- Rodrigues, M.A.M.; Souza, C.E.A.; Martins, J.A.M.; Rego, J.P.A.; Oliveira, J.T.A.; Domont, G.; Nogueira, F.C.S.; Moura, A.A. Seminal plasma proteins and their relationship with sperm motility in Santa Ines rams. Small Rumin. Res. 2013, 109, 94–100.
- Iwamoto, T.; Gagnon, C. A human seminal plasma protein blocks the motility of human spermatozoa. J. Urol. 1988, 140, 1045–1048.
- Park, K.H.; Kim, B.J.; Kang, J.; Nam, T.S.; Lim, J.M.; Kim, H.T.; Park, J.K.; Kim, Y.G.; Chae, S.W.; Kim, U.H. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 2011, 4, ra31.
- Dziewulska, K. Effect of pH, osmolality and ion concentration on spermatozoa motility and composition parameters of sperm and seminal plasma in pikeperch (Sander lucioperca L.). Aquaculture 2020, 520, 735004.
- Özgür, M.E.; Maraş, Z.; Erdoğan, S. The relationship between semen seminal plasma ions and sperm cell velocities of wild-caught longspine scraper, Capoeta trutta. Arch. Anim. Breed. 2019, 62, 557–564.
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. 2009, 29, 82–88.
- Mann, T. The Biochemistry of Semen and of the Male Reproductive Tract; John Wiley & Sons Inc.: New York, NY, USA, 1964.
- Mortimer, S.T.; Swan, M.A.; Mortimer, D. Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Hum. Reprod. 1998, 13, 2139–2146.
- Thérien, I.; Bleau, G.; Manjunath, P. Phosphatidylcholine-binding proteins of bovine seminal plasma modulate capacitation of spermatozoa by heparin. Biol. Reprod. 1995, 52, 1372–1379.
- Chiu, P.C.N.; Chung, M.-K.; Tsang, H.-Y.; Koistinen, R.; Koistinen, H.; Seppala, M.; Lee, K.-F.; Yeung, W.S.B. Glycodelin-S in Human Seminal Plasma Reduces Cholesterol Efflux andInhibits Capacitation of Spermatozoa. J. Biol. Chem. 2005, 280, 25580–25589.
- Sharkey, D.J.; Macpherson, A.M.; Tremellen, K.P.; Robertson, S.A. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol. Hum. Reprod. 2007, 13, 491–501.
- Bischof, R.J.; Lee, C.S.; Brandon, M.R.; Meeusen, E. Inflammatory response in the pig uterus induced by seminal plasma. J. Reprod. Immunol. 1994, 26, 131–146.
- Denison, F.C.; Grant, V.E.; Calder, A.A.; Kelly, R.W. Seminal plasma components stimulate interleukin-8 and interleukin-10 release. Mol. Hum. Reprod. 1999, 5, 220–226.
- Redgrove, K.A.; Nixon, B.; Baker, M.A.; Hetherington, L.; Baker, G.; Liu, D.-Y.; Aitken, R.J. The Molecular Chaperone HSPA2 Plays a Key Role in Regulating the Expression of Sperm Surface Receptors That Mediate Sperm-Egg Recognition. PLoS ONE 2012, 7, e50851.
- Bandivdekar, A.H.; Gopalkrishnan, K.; Sheth, A.R. Antibodies to human seminal plasma inhibin cause sperm agglutination and impairment of cervical mucus penetration and sperm-egg attachment. Adv. Contracept. 1987, 3, 1–12.
- Gomes, F.P.; Diedrich, J.K.; Saviola, A.J.; Memili, E.; Moura, A.A.; Yates, J.R., 3rd. EThcD and 213 nm UVPD for Top-Down Analysis of Bovine Seminal Plasma Proteoforms on Electrophoretic and Chromatographic Time Frames. Anal. Chem. 2020, 92, 2979–2987.
- Pini, T.; Leahy, T.; Paul de Graaf, S. Seminal plasma and cryopreservation alter ram sperm surface carbohydrates and interactions with neutrophils. Reprod. Fertil. Dev. 2018, 30, 689–702.
- Hernández, M.; Roca, J.; Calvete, J.J.; Sanz, L.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Vázquez, J.M.; Martínez, E.A. Cryosurvival and In Vitro Fertilizing Capacity Postthaw Is Improved When Boar Spermatozoa Are Frozen in the Presence of Seminal Plasma From Good Freezer Boars. J. Androl. 2007, 28, 689–697.
- Gómez-Fernández, J.; Gómez-Izquierdo, E.; Tomás, C.; González-Bulnes, A.; Sánchez-Sánchez, R.; de Mercado, E. Inclusion of seminal plasma in sperm cryopreservation of Iberian pig. Anim. Reprod. Sci. 2012, 130, 82–90.
- Okazaki, T.; Shimada, M. New strategies of boar sperm cryopreservation: Development of novel freezing and thawing methods with a focus on the roles of seminal plasma. Anim. Sci. J. 2012, 83, 623–629.
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729–736.
- Jones, R.E.; Lopez, K.H. Chapter 9—Gamete Transport and Fertilization. In Human Reproductive Biology, 4th ed.; Jones, R.E., Lopez, K.H., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 159–173.
- Zhao, J.; Dong, X.; Hu, X.; Long, Z.; Wang, L.; Liu, Q.; Sun, B.; Wang, Q.; Wu, Q.; Li, L. Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 22386.
- Behne, D.; Gessner, H.; Wolters, G.; Brotherton, J. Selenium, rubidium and zinc in human semen and semen fractions. Int. J. Androl. 1988, 11, 415–423.
- Ricardo, L. Male Accessory Glands and Sperm Function. Spermatozoa-Facts Perspect 2018, 101–116.
- Yeung, C.H.; Cooper, T.G.; De Geyter, M.; De Geyter, C.; Rolf, C.; Kamischke, A.; Nieschlag, E. Studies on the origin of redox enzymes in seminal plasma and their relationship with results of in-vitro fertilization. Mol. Hum. Reprod. 1998, 4, 835–839.
- Hermund, D.B. 10—Antioxidant Properties of Seaweed-Derived Substances. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 201–221.
- Candenas, L.; Chianese, R. Exosome Composition and Seminal Plasma Proteome: A Promising Source of Biomarkers of Male Infertility. Int. J. Mol. Sci. 2020, 21, 7022.
- Ortiz, W.G.; Rizo, J.A.; Carvalheira, L.R.; Ahmed, B.M.S.; Estrada-Cortes, E.; Harstine, B.R.; Bromfield, J.J.; Hansen, P.J. Effects of intrauterine infusion of seminal plasma at artificial insemination on fertility of lactating Holstein cows. J. Dairy Sci. 2019, 102, 6587–6594.
- Watson, J.G.; Carroll, J.; Chaykin, S. Reproduction in mice: The fate of spermatozoa not involved in fertilization. Gamete Res. 1983, 7, 75–84.
- Chicea, R.; Ispasoiu, F.; Focsa, M. Seminal plasma insemination during ovum-pickup--a method to increase pregnancy rate in IVF/ICSI procedure. A pilot randomized trial. J. Assist. Reprod. Genet. 2013, 30, 569–574.
- Hart, R.; Norman, R.J. The longer-term health outcomes for children born as a result of IVF treatment: Part I--General health outcomes. Hum. Reprod. Update 2013, 19, 232–243.
- Conroy, H.; Marshall, N.A.; Mills, K.H. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene 2008, 27, 168–180.
- Saccone, G.; Di Spiezio Sardo, A.; Ciardulli, A.; Caissutti, C.; Spinelli, M.; Surbek, D.; von Wolff, M. Effectiveness of seminal plasma in in vitro fertilisation treatment: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 220–225.
- Ata, B.; Abou-Setta, A.M.; Seyhan, A.; Buckett, W. Application of seminal plasma to female genital tract prior to embryo transfer in assisted reproductive technology cycles (IVF, ICSI and frozen embryo transfer). Cochrane Database Syst. Rev. 2018, 2, Cd011809.
- Adefuye, A.; Katz, A.A.; Sales, K.J. The regulation of inflammatory pathways and infectious disease of the cervix by seminal fluid. Pathol. Res. Int. 2014, 2014, 748740.
- Ceballos, A.; Remes Lenicov, F.; Sabatté, J.; Rodríguez Rodrígues, C.; Cabrini, M.; Jancic, C.; Raiden, S.; Donaldson, M.; Agustín Pasqualini, R., Jr.; Marin-Briggiler, C.; et al. Spermatozoa capture HIV-1 through heparan sulfate and efficiently transmit the virus to dendritic cells. J. Exp. Med. 2009, 206, 2717–2733.
- Stax, M.J.; van Montfort, T.; Sprenger, R.R.; Melchers, M.; Sanders, R.W.; van Leeuwen, E.; Repping, S.; Pollakis, G.; Speijer, D.; Paxton, W.A. Mucin 6 in seminal plasma binds DC-SIGN and potently blocks dendritic cell mediated transfer of HIV-1 to CD4(+) T-lymphocytes. Virology 2009, 391, 203–211.
- Velando, A.; Torres, R.; Alonso-Alvarez, C. Avoiding bad genes: Oxidatively damaged DNA in germ line and mate choice. BioEssays 2008, 30, 1212–1219.
- Amiri, I.; Sheikh, N.; Najafi, R. Nitric oxide level in seminal plasma and its relation with sperm DNA damages. Iran. Biomed. J. 2007, 11, 259–264.
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37.
- Schaad, N.C.; Zhang, X.Q.; Campana, A.; Schorderet-Slatkine, S. Human seminal plasma inhibits brain nitric oxide synthase activity. Hum. Reprod. 1996, 11, 561–565.
