Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell & Tissue Engineering
Small extracellular vesicles (sEV) are a group of particles of nanometric size, released by virtually all cells. These vesicles function as biologic messengers, carrying proteins, lipids and nucleic acids as a form of paracrine cellular communication. Umbilical cord blood (UCB) is a priviledged source of sEV, due to its abundance in naïve cells with strong regenerative and immunomodulatory potential. Hence, UCB-derived sEV can be exploited for their therapeutic properties, taking advantage of nature's tools for achieving homeostasis.
- extracellular vesicles
- EV
- umbilical cord blood
1. Introduction
Virtually every living cell releases extracellular vesicles (EV), which can be classified based on size and marker expression. One of the smallest known groups of EV, exosomes, have a diameter typically ranging from 30 to 100 nm and originate from endosomes [1]. Composed of lipids, proteins and nucleic acids, these ubiquitous vesicles are thought to be involved in multiple diseases, including inflammatory and autoimmune skin conditions [2]. Due to their physical characteristics, which allow them to carry molecules across long distances, EV are often explored for their potential as biomarkers [3,4]. Physiologically, small EV (sEV), such as exosomes, are key mediators of cellular communication, namely through microRNAs [5]. Hence, depending on the producing cell, sEV may have modulating characteristics, which can potentially be harnessed for therapeutic purposes. In fact, these naturally-produced vesicles are currently explored for the treatment of several conditions, including wound healing [6] and autoimmune diseases [7,8,9]. Their use replaces cell therapies [10,11], while conferring advantages, namely concerning handling and formulation.
Umbilical cord blood (UCB) is a rich source of stem cells and immature T-cells [16] with potent suppressive ability [17]. The collection of UCB, commonly seen as medical waste, is non-invasive and has limited ethical concerns. It has previously been shown that sEV from UCB mononuclear cells (UCB-MNC-sEV), produced using an established optimized protocol [18], accelerate the healing of diabetic wounds [6] and have a good safety profile [19].
2. Current Insight on Umbilical Cord Blood-Derived Small Extracellular Vesicles
Over the last years, sEV have been explored for their potential as cell-free immunomodulatory and regenerative agents. Indeed, unmodified or engineered sEV were shown to have therapeutic potential across multiple conditions, including cancer [23], inflammatory lung diseases [24,25], and autoimmunity [26]. Here, we explore the mechanism of action of sEV isolated from UCB-MNC, and evaluate their effect in psoriasis models.
In vitro, UCB-MNC-sEV exhibit anti-inflammatory properties, affecting macrophage differentiation and cytokine production. These results are consistent with previous findings using sEV isolated from bone marrow [20] or cord blood [21]. We also show that the presence of UCB-MNC-sEV-induced M2 macrophages has a down-stream effect on neighboring cells, such as skin fibroblasts, reducing their response to an inflammatory trigger. Moreover, UCB-MNC-sEV strongly inhibited cell proliferation and cytokine production by LPS-stimulated total CD4+ and CD8+ T-cells, consistent with previous reports [11,27]. This outcome is possibly due to an effect on T-cell differentiation, given that UCB-MNC-sEV promote a shift from a Th1 or Th17 into a Treg phenotype. Notably, UCB-MNC-sEV stimulus was shown to be as effective as IL-2 in promoting Treg development.
Our in vitro findings evidenced a potential mechanism of action for UCB-MNC-sEV, responsible for a shift in the expression of transcription factors, which favor Treg differentiation and concomitantly silence Th17 signaling. Biologics targeting the Th17 axis (α-IL-17 and α-IL-23) have been proved to be clinically effective in ameliorating psoriasis symptoms [28,29,30]. Additionally, previous reports suggest that Treg, a typically tolerogenic cell population, play a crucial role in the maintenance of skin homeostasis. Treg-deficient animals present an exacerbated response to imiquimod [31] and Treg from psoriatic patients display an impaired suppressive function [32]. Hence, we hypothesized that UCB-MNC-sEV’s profile could be therapeutically beneficial in psoriasis. To test this, we employed a model of reconstructed human epidermis, composed of keratinocytes in various stages of differentiation, and pre-treated to display psoriasis-like inflammatory features. UCB-MNC-sEV treatment significantly reduced the expression of psoriasis-associated molecules, including IL-6 and IL-8, as well as antimicrobial peptides S100A7 and DEFB4, thereby supporting its therapeutic potential for this disease.
In order to test UCB-MNC-sEV’s effect in vivo, we first designed a micelle-rich hydrogel that solidifies at normal body temperature, thus reducing product loss when applied to the skin. Psoriasis-like symptoms were induced by topical applications of imiquimod, and hydrogel was applied 1 h later, alone or containing UCB-MNC-sEV. In this in vivo model, UCB-MNC-sEV proved superior to hydrogel in reducing or preventing keratinocyte hyperproliferation, as measured by epidermal thickness. Yet, there were no significant differences in the disease scores, mRNA expression and cellular profile between the two treatment groups. While it is possible that the application of hydrogel alone strongly improves psoriatic symptoms, the results observed are better explained by the possible trapping of imiquimod molecules in hydrogel micelles, therefore preventing full symptom development. An alternative experimental setting would either allow for a longer time interval between imiquimod and test treatment applications and/or require the increase of imiquimod dosage. Nevertheless, in line with previous in vitro data, UCB-MNC-sEV had a positive effect on keratinocytes and were tendentially stronger than hydrogel alone in shifting skin cellular infiltrates toward a tolerogenic profile. Importantly, gene expression data from chronic wounds support the increase in Treg differentiation following UCB-MNC-sEV treatment. Given the incomplete therapeutic response of imiquimod-treated animals to UCB-MNC-sEV, it is plausible that UCB-MNC-sEV could act as an adjuvant treatment, in combination with standard therapies, such as anti-IL-17A or anti-IL23. This strategy would not only target immune-driven disease pathways, but also simultaneously stimulate repair mechanisms in the skin.
In conclusion, we show that UCB-MNC-sEV decrease inflammation by targeting different cell populations, such keratinocytes, fibroblasts, and macrophages, and by modulating T-cell differentiation and cytokine production (Figure 1). These findings warrant further proof-of-concept studies on the therapeutic potential of UCB-MNC-sEV in inflammatory skin conditions, in particular diseases thought to benefit from Th17/Treg-targeting.
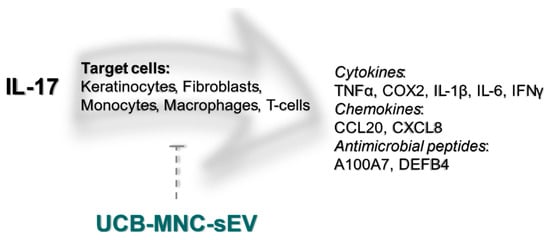
Figure 1. UCB-MNC-sEV’s putative effect on the IL-17 signaling pathway. IL-17, particularly IL-17A, is thought to be a crucial driver of psoriatic disease. IL-17 target cells include keratinocytes, fibroblasts, monocytes, macrophages, and T-cells, all of which are likewise targeted by UCB-MNC-sEV, either directly or indirectly. As shown in this paper, treatment with UBC-MNC-sEV affects multiple players of the IL-17 cascade, and results in a lower expression and/or release of psoriasis-associated mediators, such as TNFα, COX2, IL-1β, IL-6, IFNγ, CCL20, CXCL8, A100A7 and DEFB4.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22189797
This entry is offline, you can click here to edit this entry!
