Harmful cyanobacterial blooms pose an environmental health hazard due to the release of water-soluble cyanotoxins. One of the most prevalent cyanotoxins in nature is microcystins (MCs), a class of cyclic heptapeptide hepatotoxins, and they are produced by several common cyanobacteria in aquatic environments. Once released from cyanobacterial cells, MCs are subjected to physical chemical and biological transformations in natural environments. MCs can also be taken up and accumulated in aquatic organisms and their grazers/predators and induce toxic effects in several organisms, including humans.
- microcystins
- hepatotoxin
- cyanotoxins
1. Introduction
Microcystins (MCs) are a class of liver toxins that are toxic to humans and animals, alike [1]. MCs are produced as secondary metabolites by a number of widely distributed freshwater cyanobacteria, including Microcystis , Planktothrix , Anabaena, and Oscillatoria genera [2]. Once synthesized, MCs are stored intracellularly and only released into the water following cell lysis, either by viral infection or during cell senescence [3]. During growth seasons, MCs are often measured at concentrations that exceed the guideline values published by the World Health Organization for safe use for drinking (>1 µg/L) and recreational purposes (>20 µg/L for moderate probability for adverse health effects) in freshwaters across the world, even in some of the largest lakes, like Lake Erie [4] (United States) and Lake Taihu [5] (China).
Several MC research, including chemical structures, detection [6][7], ecological impacts [8], human health risk [9], mechanism of toxicity [10][11], synthesis [12], degradation/removal pathways [13][14], and more. Among these different aspects, cellular toxicity has been relatively under studied, which has been addressed in this review. However, a review that encompasses the multifaceted features of MC research is lacking, which is necessary to yield a comprehensive understanding of the health effects and ecosystem impact of MCs.
MCs congeners share a common cyclic structure that is formed by seven amino acid residues, cyclo-(D-Ala 1)-X 2-(D-MeAsp 3)-Z 4-Adda 5-(D-Glu 6)-Mdha 7 [15] ( Figure 1 ). The percentage variation for the amino acid residue has been depicted in Figure 1 . The two L-amino acids of MCs at positions 2 and 4, i.e., X 2 and Z 4, are the most variable by substitution and account for the most diversity of MC congeners [15]. The structures of the rest of the amino acid residues are largely constant, although variations at each of these positions have been reported ( Figure 1 ). The number of identified MC congeners has been consistently increasing and reached 279 very recently [16]; more are expected to be discovered [17].
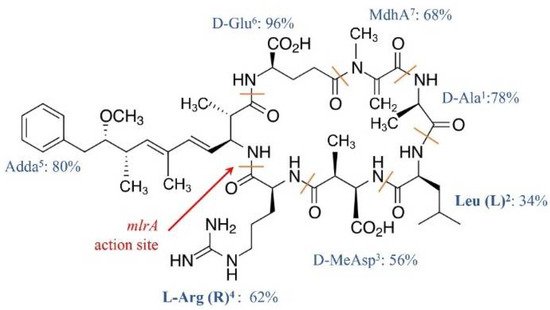
Ala 1, MeAsp 3, and Glu 6 were D-amino acids. Adda 5, or C 20 amino acid (3-amino-9-methoxy-2, 6, 8-trimethyl-10-phenyldeca-4, 6-dienoic acid), and MdhA 7 are non-proteinogenic amino acids; they contribute significantly to the toxicity of MCs and are also found in the structure of another cyanotoxin, i.e., nodularin [18].
2. Exposure and Toxicology
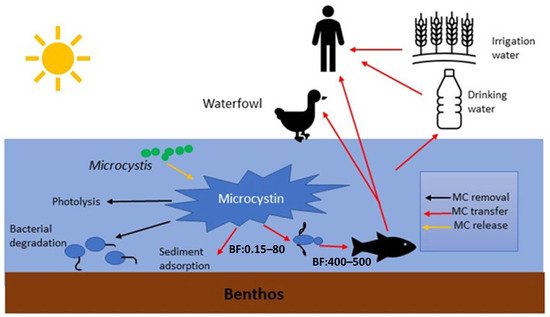
MCs in natural waters can affect humans via various routes, including chronic and accidental ingestion of contaminated drinking or recreational water, inhalation or contact with the nasal mucous membrane, dermal contact with toxins during recreational activities, and consumption of contaminated food irrigated with (vegetables, fruits) or grown in (i.e., fish and shellfish) contaminated water [19] ( Figure 2 ).
Developing a better understanding of the toxicity of MCs will enable us to assess the risk of exposure to these commonly encountered cyanotoxins. The primary cytotoxicity of MCs is the inhibition of Protein Phosphatases 1 and 2a (PP1 and 2a), which leads to several subsequent harmful effects. Acute exposure via ingestion of MCs by humans at concentrations over 10 µg /L can cause various symptoms, including vomiting, diarrhea, abdominal pain, and blistering around the mouth, or it can even ultimately lead to liver failure [20]. However, MCs are rarely ingested directly at acute lethal doses by humans. Chronic and frequent exposure to MCs at low concentrations can ultimately lead to liver failure due to chronic liver cell apoptosis or uncontrolled cell proliferation, leading to primary liver cancer [21][22][23].
Once they have gained exposure to animal cells, MCs first get absorbed into the intestinal tract and then earn entry to the blood stream, where they can be distributed to a range of organs [24]. MCs enter human cells via transmembrane organic anion transporter peptides (OATPs) (Fischer et al., 2005). Receptors of OATPs are abundant on the hepatic cells, making liver a primary target for MCs; about 50–70% of MCs in blood streams can be taken up by the liver [25]. With less abundance, OATPs also exist on cells of other organs, such as kidney and brain cells, making them also susceptible to MC toxicity [25]. In comparison with hepatic cells, non-hepatic cells require a greater MC dose and longer exposure time for cell death to occur [10][26]. When being exposed to the same concentrations of MC-LR (0.8 µM), hepatocytes were found to shrink and lose their viability (dying) within 30 min, while endothelial, fibroblasts, and epithelial cells remained viable for up to 5 days [26].
The final stage of MC intoxication may include cell death due to apoptosis or necrosis 27; the former mechanism has been found to be more common [10]. Many studies found that MCs can induce apoptosis via the intrinsic pathway, starting with the production of reactive oxidative species (ROS), which then increase mitochondrial permeability potential (MPP) followed by the induction of caspase 9 and then 3 in that sequence [27][28][29]. A few recent studies have also suggested the importance of extrinsic apoptosis, which starts with the Fas/Fas-L receptor, followed by the induction of caspase 8, followed by the induction of caspase 3 [30][31][32]. MC can also induce apoptosis via activation of the NF-kB pathway (part of the extrinsic pathway) followed by caspase 9 induction [33] in INS-1 cells, which involves both extrinsic and intrinsic pathways. A recent work has shown the induction of caspase 8 before the induction of caspase 9 when we have a combination of extrinsic and intrinsic pathways in MC-treated hepatic cells [32].
3. Production of MCs and Their Regulating Factors
MC synthesis in cyanobacteria is carried out by a gene cluster that possesses eight genes of a total of over 55 kbp DNA ( mcyA-J ), which encode 48 catalytic reactions [34]. A number of precursors are incorporated during the MC synthesis, including phenylacetate, malonyl-CoA, SAM (S-adenosyl methionine), glutamate, serine, alanine, leucine, D -methyl-iso-aspartate, and arginine [34][35]. The arrangements and sequences of mcy genes in the genome and their products (enzymes/proteins) differ among cyanobacterial species [36] (12); therefore, the prevalence of MC congeners during cyanobacterial blooms is determined by dominant cyanobacterial species [17].
Besides inherent genetic properties, a number of environmental factors can impact MC synthesis. Higher dissolved oxygen in water has been found to increase MC production [37][38][39]. High nitrogen concentrations have been found to limit the amount of MCs produced by Microcystis aeruginosa , while high sulfur and phosphorous supplies behave the opposite [40][41]. Solar irradiance, and UV-B intensities, in particular, has been found to encourage the growth of MC-producing cyanobacteria over non-toxin producing strains [42]. Solar irradiance and nutrient supplies together have also been found to impact not only the amount but also the types of MC congeners produced by Microcystis aeruginosa and Planktothrix agardhii [43][44]. Specifically, these cyanobacteria produced higher amounts of MCs with more toxic variants, like MC-LR, than MC-RR under high solar irradiance and nutrient supply. However, the above observations can be species-specific, as MC productions were not altered by light for M. wesenbergii and Aphanizomenon aphanizomenoides under varied light and nutrients [45].
4. Fate of Extracellular MCs in Environments
The stable ring structure of MCs protects them from proteases that are commonly found in environments and a group of enzymes that is responsible for degradation of many organic compounds [46][47][48].
Most MC-degrading bacteria are isolated from aerobic environments, but studies have also obtained MC degraders from oxygen-limited environments, such as alphaproteobacteria and gammproteobacteria from drinking water sludge [49], deltaproteobacteria from the mucilage of Microcystis cells [50], and commercially available probiotic bacteria [51], which belong to Proteobacteria (alpha, beta, and gamma), Actinobacteria, and Firmicutes.
The rate of microbial degradation of MCs is affected by a number of abiotic factors, including temperature, pH, DOC (dissolved organic carbon), and nutrient availability [52]. Temperature at the 30–40 °C range has been found to favor MC biodegradation [53][54][55]. Temperatures that are lower or higher (±10–20 °C) than this range can slow down the degradation of MCs [54].
Organic nutrients (C.N and P) are commonly found in excess in eutrophic lakes, especially during CyanoHABs [56]. Many studies have revealed that a supply of dissolved organic nutrients may slow down MC degradation [57][52]. However, a few recent studies have found improved or unchanged microcystin degradation by certain bacteria species after additions of an organic C source [53][58].
Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/w13162147
References
- Labine, M.; Minuk, G. Cyanobacterial toxins and liver disease. Can. J. Physiol. Pharmacol. 2009, 87, 773–788.
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20.
- Graham, L.; Loftin, K.; Meyer, M.; Ziegler, A. Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern United States. Environ. Sci. Technol. 2010, 19, 7361–7368.
- Michalak, A.; Anderson, E.; Beletsky, D.; Boland, S.; Bosch, N.; Bridgeman, T.; Chaffin, T.; Cho, K.; Confesor, R.; Daloglu, I. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452.
- Sakai, H.; Hao, A.; Iseri, Y.; Wang, S.; Kuba, T.; Zhang, Z.; Katayama, H. Occurrence and distribution of microcystins in Lake Taihu, China. Sci. World J. 2013, 2013, 1–7.
- Hawkins, P.R.; Novic, S.; Cox, P.; Neilan, B.A.; Burns, B.P.; Shaw, G.; Saitou, T. A review of analytical methods for assessing the public health risk from microcystin in the aquatic environment. J. Water Supply Res.Technol. 2005, 54, 509–518.
- Sangolkar, L.N.; Maske, S.S.; Chakrabarti, T. Methods for determining microcystins (peptide hepatotoxins) and microcystin-producing cyanobacteria. Water Res. 2006, 40, 3485–3496.
- Machado, J.; Campos, A.; Vasconcelos, V.; Freitas, M. Effects of microcystin-LR and cylindrospermopsin on plant-soil systems: A review of their relevance for agricultural plant quality and public health. Environ. Res. 2017, 153, 191–204.
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in estuarine and marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45.
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287.
- Lone, Y.; Kohli, R.; Bhide, M. An overview of the toxic effect of potential human carcinogen MC-LR on testis. Toxicol. Rep. 2015, 2, 289–296.
- Christiansen, G.; Fastner, J.; Erhard, M.; Börner, T.; Dittmann, E. Microcystin biosynthesis in Planktothrix: Genes, evolution, and manipulation. J. Bacteriol. Res. 2003, 185, 564–572.
- Westrick, J.A.; Szlag, D.C.; Southwell, B.J.; Sinclair, J. A review of cyanobacteria and cyanotoxins removal/inactivation in drinking water treatment. Analyt. Bioanalyt. Chem. 2010, 397, 1705–1714.
- Li, J.; Li, R.; Li, J. Current research scenario for microcystins biodegradation—A review on fundamental knowledge, application prospects and challenges. Sci. Total Environ. 2017, 595, 615–632.
- Du, X.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiwicz, M.; Guo, H.; Zhang, H. The diversity of cyanobacterial toxins on structural characterization, distribution, and identification: A systematic review. Toxins 2019, 11, 530.
- Bouaicha, N.; Miles, C.; Beach, D.; Labidi, Z.; Djabri, A.; Benayache, N.; Nguyen-Quang, T. Structural diversity, characterization and toxicology of microcystins. Toxins 2019, 11, 714.
- Diez-Quijada, L.; Prieto, A.; Guzman-Guilllen, R.; Jos, A.; Camean, A. Occurrence and toxicity of microcystin congeners other than MC-LR and MC-RR: A review. Food Chem. Toxicol. 2019, 125, 106–132.
- He, X.; Stanford, B.; Adams, C.; Rosenfield, E.; Wert, E. Varied influence of microcystin structural difference on ELISA cross-reactivity and chlorination efficiency of congener mixtures. Water Res. 2017, 126, 515–523.
- Poste, A.; Hecky, R.; Guildford, S. Evaluating MC Exposure Risk through Fish Consumption. Environ. Sci. Technol. 2011, 45, 5806–5811.
- Blaha, L.; Babica, P.; Marsalek, B. Toxins produced in cyanobacterial water blooms-toxicity and risks. Interdiscip. Toxicol. 2009, 2, 36–41.
- McLellan, N.; Manderville, R. Toxic mechanisms of microcystins in mammals. Toxicol. Res. 2017, 6, 391–405.
- Woolbright, B.; Williams, D.; Ni, H.; Kumer, S.; Schmitt, T.; Kane, B.; Jaeschke, H. Microcystin-LR induced liver injury in mice and in primary human hepatocytes is caused by oncotic necrosis. Toxicon 2017, 125, 99–109.
- Ong, M.M.; Deng, S.; Halim, C.; Cai, W.; Huang, R.; Sethi, G.; Hooi, S.; Kumar, A.; Yap, C. Cytoskeletal proteins in cancer and intracellular stress: A therapeutic perspective. Cancers 2020, 12, 238.
- Meier-Abt, F.; Hammann-Hanni, A.; Stieger, B.; Ballatori, N.; Boyer, J. The organic anion transport polypeptide 1d1 (Oatp1d1) mediates hepatocellular uptake of phalloidin and microcystin into skate liver. Toixcol. Appl. Pharmacol. 2007, 218, 274–279.
- Fischer, W.; Altheimer, S.; Cattori, V.; Meier, P.; Dietrich, D.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263.
- McDermott, C.; Nho, C.; Howard, W.; Holton, B. The cyanobacterial toxin, MC-LR, can induce apoptosis in a variety of cell types. Toxicon 1998, 36, 1981–1986.
- Weng, D.; Yan, L.; Yinna, W.; Ying, L.; Pingping, S. The Role of ROS in MC-LR-induced Hepatocyte Apoptosis and Liver Injury in Mice. Toxicology 2007, 232, 15–23.
- Li, Y.; Shao, J.; Wu, X.; Xu, Y.; Li, R. MC production by Microcystis aeruginosa: Direct regulation by multiple environmental factors. FEMS Microbiol. Lett. 2011, 322, 108–114.
- Huang, H.; Liu, C.; Fu, X.; Zhang, S.; Xin, Y.; Li, Y.; Xue, L.; Cheng, X.; Zhang, H. Microcystin-LR induced apoptosis in rat sertoli cells via the mitochondrial caspase-dependent pathway: Role of reactive oxygen species. Front. Physiol. 2016, 7, 397–412.
- Zhao, Y.; Li, R.; Xia, W.; Neuzil, J.; Lu, Y.; Zhang, H.; Zhao, X.; Zhang, X.; Sun, C.; Wu, K. Bid integrates intrinsic and extrinsic signaling in apoptosis induced by α-tocopheryl succinate in human gastric carcinoma cells. Cancer Lett. 2010, 288, 42–49.
- Wang, M.; Su, P. The role of the Fas/FasL signaling pathway in environmental toxicant-induced testicular cell apoptosis: An update. Syst. Biol. Reprod. Med. 2017, 64, 93–102.
- Krishnan, A.; Koski, G.; Mou, X. Characterization of microcystin-induced apoptosis in HepG2 hepatoma cells. Toxicon 2019, 173, 20–26.
- Feng, G.; Abdalla, M.; Li, Y.; Bai, Y. NF-kB mediates the induction of Fas receptor and Fas ligand by microcystin-LR in HepG2 cells. Mol. Cell Biochem. 2011, 352, 209–219.
- Tillett, D.; Dittmann, E.; Erhard, M.; von Döhren, H.; Börner, T.; Neilan, B.A. Structural organization of MC biosynthesis in Microcystis aeruginosa PCC7806: An integrated peptide–polyketidesynthetase system. Chem. Biol. 2000, 7, 753–764.
- Dittmann, E.; Neilan, B.; Erhard, M.; Dohren, H.; Borner, T. Insertional mutagenesis of a peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol. Microbiol. 1997, 26, 779–787.
- Rouhiainen, L.; Vakkilainen, T.; Siemer, B.L.; Buikema, W.; Haselkorn, R.; Sivonen, K. Genes coding for hepatotoxic heptapeptides (microcystins) in the cyanobacterium Anabaena strain 90. Appl. Environ. Microbiol. 2004, 70, 686–692.
- Wicks, R.; Thiel, P. Environmental factors affecting the production of peptide toxins in floating scums of the cyanobacterium Microcystis aeruginosa in a hypertrophic African Reserve. Environ. Sci. Technol. 1990, 24, 1413–1418.
- Srivastav, A.; Choi, G.; Ahn, C.; Oh, H.; Ravi, A.; Asthana, R. dynamics of microcystin production and quantification of potentially toxigenic microcystis sp. using real-time PCR. Water Res. 2012, 46, 817–827.
- Kotak, B.; Lam, A.K.Y.; Prepas, A.E.; Hrudey, S. Role of chemical and physical variables in regulating MC-LR concentration in phytoplankton of eutrophic lakes. Can. J. Fisher Aqu. Sci. 2000, 57, 1584–1593.
- Oh, H.; Le, S.; Jang, M.; Yoon, B. MC Production by Microcystis aeruginosa in a Phosphorus-Limited Chemostat. Appl. Environ. Microbiol. 2000, 66, 176–179.
- Laszakovits, J.; MacKay, A. Removal of cyanotoxins by potassium permanganate: Incorporating competition from natural water constituents. Water Res. 2019, 155, 1–28.
- Ding, X.; Li, X.; Hong-Ying, D.; Ik-Kyo, C.; Jin-Ae, L. Toxic effects of microcystis cell extracts on the reproductive system of male mice. Toxicon 2006, 48, 973–979.
- Tonk, L. The MC composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity. Appl. Environ. Microbiol. 2005, 71, 5177–5181.
- Hesse, K.; Kohl, J.-G. Effects of light and nutrient supply on growth and MC content of different strains of Microcystis aeruginosa. Appl. Environ. Microbiol. 2001, 104–115.
- Xie, L.; Xie, P.; Guo, L.; Li, L.; Miyabara, Y.; Park, H. Organ distribution and bioaccumulation of MCs in freshwater fish at different trophic levels from the eutrophic Lake Chaohu, China. Environ. Toxicol. 2005, 20, 293–300.
- Tsuji, K.; Naito, S.; Kondo, F.; Ishikawa, N.; Watanabe, M.; Suzuki, M.; Harada, K. Stability of MCs from cyanobacteria: Effect of light on decomposition and isomerization. Environ. Sci. Technol. 1994, 28, 173–177.
- Harada, K.M.F.; Watanabe, K.I.; Harada, W.W.C.; Fujiki, H. Chemistry and Detection of MCs in Toxic MCs; CRC Press: Boca Raton, FL, USA, 1996; pp. 103–143.
- Jones, G.J.; Bourne, D.G.; Blakeley, R.L.; Doelle, H. Degradation of the cyanobacterial hepatotoxin microcystin by aquatic bacteria. Nat. Toxins 1994, 2, 228–235.
- Ma, G.; Pei, H.; Hu, W.; Xu, X.; Ma, C.; Li, X. The removal of cyanobacteria and their metabolites through anoxic biodegradation in drinking water sludge. Biores. Technol. 2014, 165, 191–198.
- Maruyama, T.; Kato, K.; Yokoyama, A.; Tanaka, T.; Hiraishi, A.; Park, H.-D. Dynamics of MC-degrading bacteria in mucilage of Microcystis. Microb. Ecol. 2003, 46, 279–288.
- Nybom, S.; Salminen, S.; Meriluoto, J. Removal of MC-LR by strains of metabolically active probiotic bacteria. FEMS Microbiol. Lett. 2007, 270, 27–33.
- Phujomjai, Y.; Somdee, A.; Somdee, T. Biodegradation of MC [Dha7] MC-LR by a novel MC-degrading bacterium in an internal airlift loop bioreactor. Water Sci. Technol. 2013, 73, 267–274.
- Krishnan, A.; Zhang, Y.; Balaban, M.; Seo, Y.; Mou, X. Taxonomical and genotypical heterogeneity of microcystin degrading bacterioplankton in western Lake Erie. Harmful Algae 2020, 98, 1–10.
- Yang, F.; Masssey, I.; Guo, J.; Yang, S.; Pu, Y.; Zeng, W.; Tan, H. Microcystin-LR degradation utilizing a novel effective indigenous bacterial community YFMCD1 from Lake Taihu. J. Toxicol. Environ. Health Part. A 2018, 81, 1–10.
- Okano, K.; Shimizu, K.; Kawauchi, Y.; Maseda, H.; Motoo, U.; Zhnag, Z.; Neilan, B.; Suguira, N. Characteristics of a microcystin-degrading bacterium under alkaline environmental conditions. J. Toxicol. 2009, 2009, 1687–1691.
- Carmichael, W. The toxins of cyanobacteria. Sci. Am. 1992, 2, 78–86.
- Wang, J.; Wang, C.; Li, Q.; Shen, M.; Bai, P.; Li, J.; Lin, Y.; Gan, N.; Li, T.; Zhao, J. Microcystin-LR Degradation and Gene Regulation of Microcystin-Degrading Novosphingobium sp. THN1 at Different Carbon Concentration. Front. Microbiol. 2019, 2019, 1740–1750.
- Li, J.; Shimizu, K.; Sakharkar, M.K.; Utsumi, M.; Zhang, Z.; Sugiura, N. Comparative study for the effects of variable nutrient conditions on the biodegradation of microcystin-LR and concurrent dynamics in microcystin-degrading gene abundance. Bioresour. Technol. 2011, 102, 9509–9517.
