Phenols are widespread in nature, being the major components of several plants and essential oils. Natural phenols’ anti-microbial, anti-bacterial, anti-oxidant, pharmacological and nutritional properties are, nowadays, well established. Hence, given their peculiar biological role, numerous studies are currently ongoing to overcome their limitations, as well as to enhance their activity.
- carvacrol
- thymol
- eugenol
- resveratrol
- hispolon
- hydroxytyrosol
- lipidic phenols
- phenolic acids
- polyphenols
- curcumin
1. Introduction
2. Tailored Functionalization of Natural Phenols to Improve Biological Activity
2.1. Monophenols
Carvacrol, thymol and eugenol are amongst the most widespread phenols in nature, usually responsible for beneficial plant properties.
Carvacrol (5‐isopropyl‐2‐methylphenol) is a phenolic monoterpenoid compound, and it is a major component of oregano and thyme essential oils. Together with its isomer, thymol (2‐isopropyl‐5‐methylphenol), it is the main active ingredient responsible for essential oils’ biological activity [82][83][84]. In fact, carvacrol’s peculiar antibacterial, antifungal, anti‐inflammatory, anxiolytic and anticancer activities are currently well established, and the FDA (Food and Drug Administration) has approved its use as an additive in food products. Nonetheless, the research of new carvacrol analogues is currently inspiring several research groups, with the aim to extend the potential application of the compound [85]. Carvacrol functionalization usually occurs at the ‐OH moiety; indeed, a wide variety of synthetic carvacryl esters can be found in the literature. Obviously, through phenol esterification, variegated functionalized products can be accessed [86], to be explored in several areas.
Next to carvacrol, its isomer, thymol, is widely used as an antibacterial, antifungal, antioxidant and anti‐inflammatory active ingredient in several products, as well as a food preservative [84][87]. Indeed, several natural and synthetic thymol derivatives have been proposed over the years to further broaden its application at the industrial level [88][89][90]. Quite a few thymol derivatives have been synthesized and evaluated for different biological purposes [86][91][92][93][94]. Thymol functionalization through esterification or etherification reactions constitutes one of the most useful approaches to access a wide library of different bio‐active molecules. Thymol esterification usually occurs in the classical conditions, reacting thymol with the appropriate anhydride or acyl chloride in the presence of a base. MW‐assisted procedures in aqueous medium have also been proposed, to perform reactions in reduced times and with improved yields [95]. Different studies also demonstrated that halogenation is a proficient strategy to enhance thymol biological activity [96][97][98][99][100][101].
Eugenol (4‐allyl‐2‐methoxyphenol) is the major component of clove essential oils, but it can be also found in minor amounts in cinnamon, clover pepper and other plants. It is used in perfumeries for its pleasant fragrance, as a flavoring agent in foods, as antiseptic and disinfectant in dental products and in many other fields [102]. Eugenol can be readily functionalized through the chemical transformation of the phenolic ‐OH group (mainly via the classical etherification and esterification reactions) [103][104][105][106][107][108][109], on the aromatic ring (through nitration reaction or Mannich bases formation) [110][111][112], as well as on the allylic functionality, through epoxidation [108].
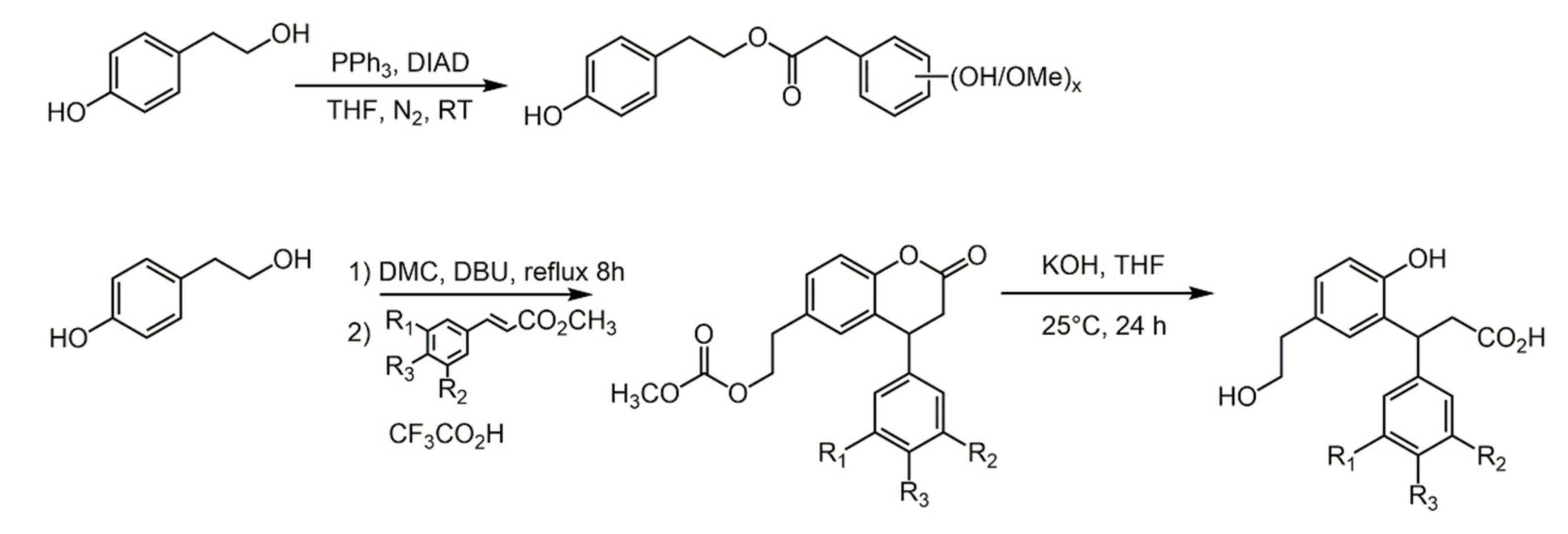
2.2. Diphenols
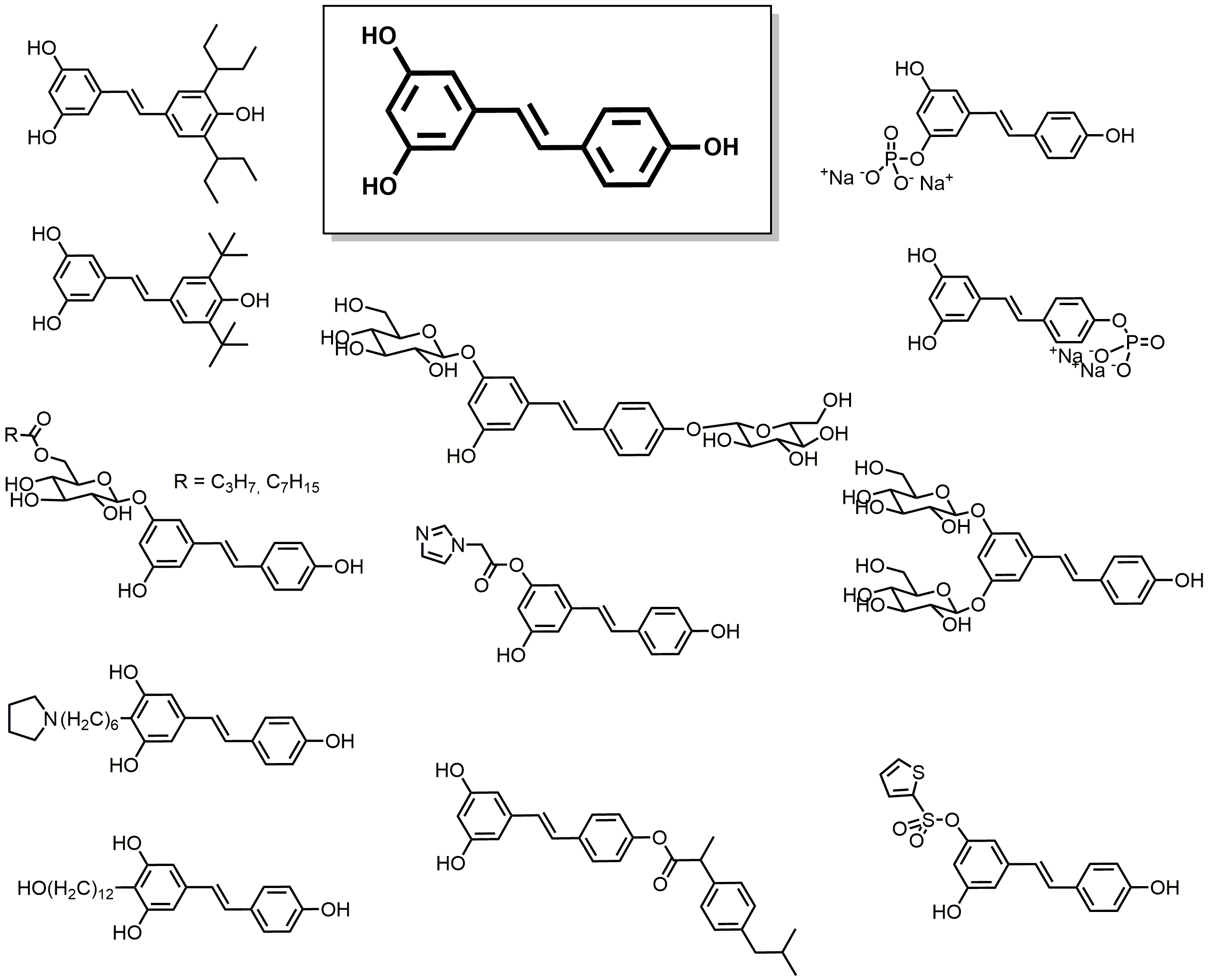
2.3. Phenolic Acids
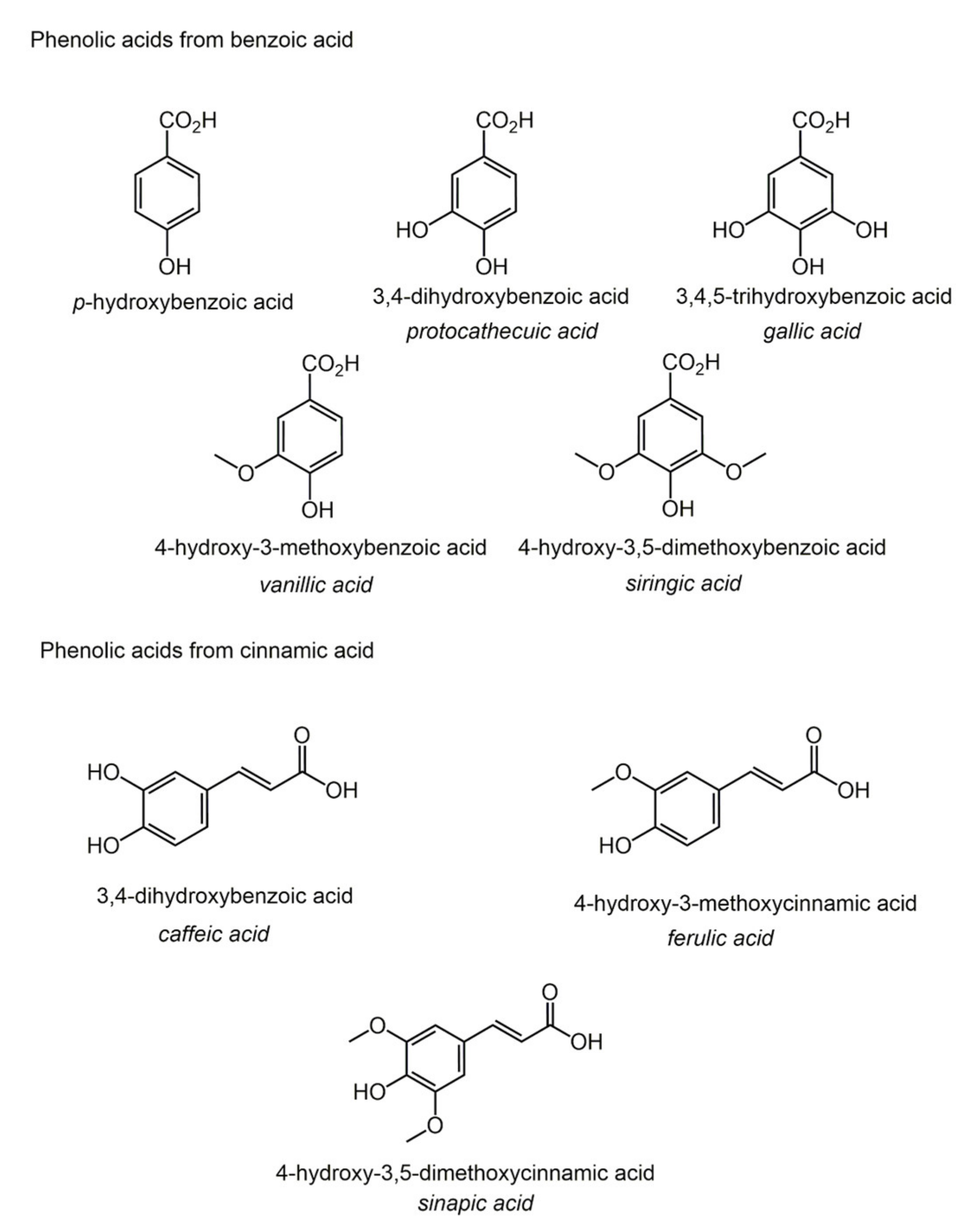
2.4. Lipidic Phenols
2.5. Polyphenols
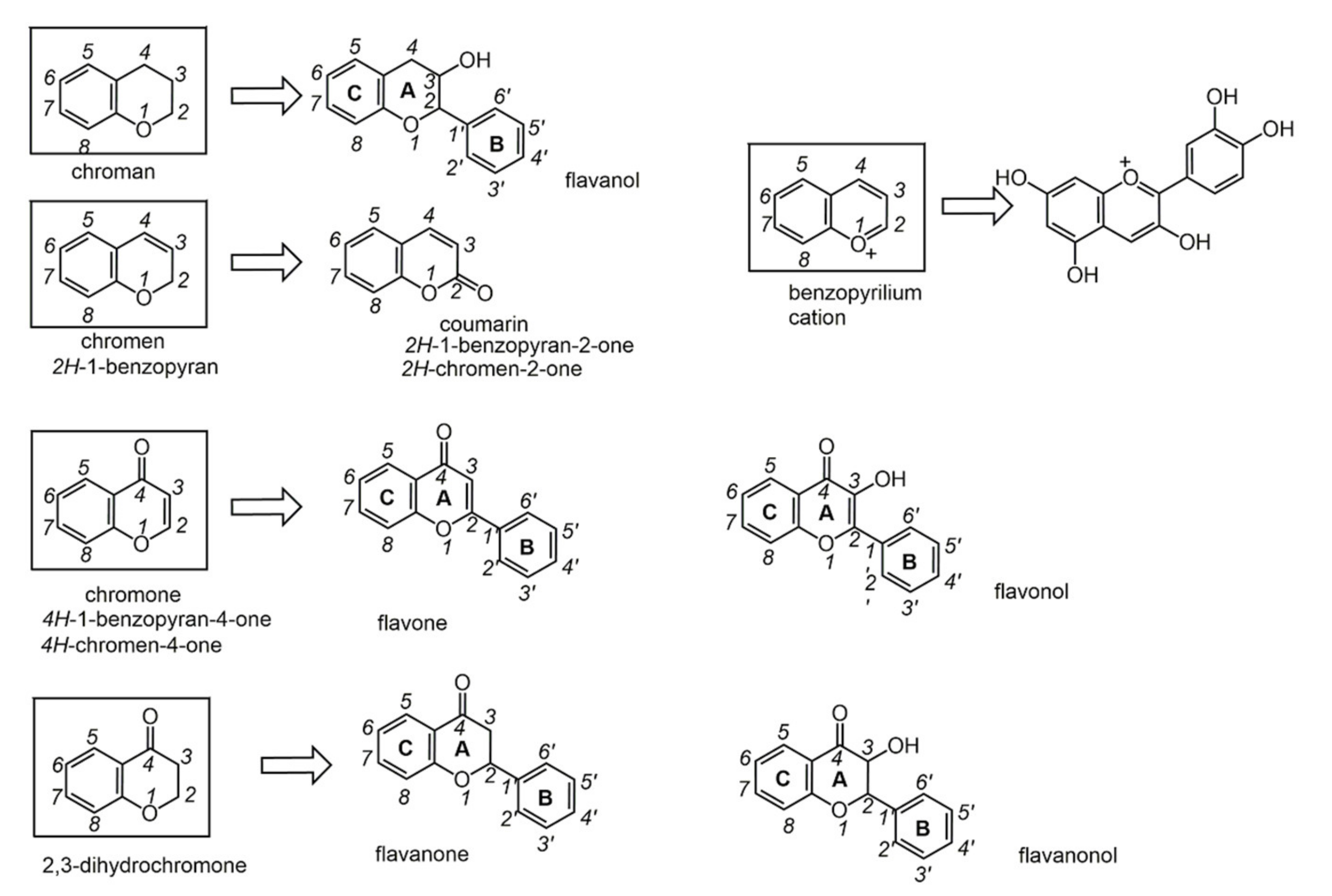
Chemical transformations of natural phenols might lead to more effective species, if structural features at the basis of biological activity are understood.
2.6. Curcumin and Curcuminoids
Curcumin, [1,7‐bis(4‐hydroxy‐3‐methoxyphenyl)‐1,6‐heptadiene‐3,5‐dione], a yellow pigment isolated from turmeric (Curcuma longa Linn), is a multifunctional compound that, at least from reading the literature of the past twenty years, seems a sort of panacea for all the illness of modern society, cancer and Alzheimer’s disease included. Phenolic –OH groups ensure the anti‐oxidant properties, whereas the extensive conjugation due to the keto‐enol equilibrium is the basis of photodynamic activity. Several recent reviews discuss the aspects of biological activity [144][145][146] and possible medical applications [147][148][149][150][151][152] of curcumins and derivatives. An increasing interest is devoted to curcumin‐based drugs against neurodegenerative diseases [153], especially Alzheimer’s [154] and cancer [155].
The quest for new curcumin derivatives is motivated by (i) the necessity of increasing the material availability, and (ii) the necessity of meliorating the solubility in aqueous solution.
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/biom11091325
References
- Staszowska-Karkut, M.; Materska, M. Phenolic composition, mineral content, and beneficial bioactivities of leaf extracts from black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463.
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664.
- Mouwakeh, A.; Kincses, A.; Nové, M.; Mosolygó, T.; Mohácsi-Farkas, C.; Kiskó, G.; Spengler, G. Nigella sativa essential oil and its bioactive compounds as resistance modifiers against Staphylococcus aureus. Phytother. Res. 2019, 33, 1010–1018.
- Chibane, L.B.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant Antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474.
- Muhammad, D.R.A.; Tuenter, E.; Patria, G.D.; Foubert, K.; Pieters, L.; Dewettinck, K. Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem. 2021, 340, 127983.
- Pavlić, B.; Tesli, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724.
- Sánchez-Gutiérrez, J.A.; Moreno-Lorenzana, D.; Álvarez-Bernal, D.; Rodríguez-Campos, J.; Medina-Medrano, J.R. Phenolic profile, antioxidant and anti-proliferative activities of methanolic extracts from Asclepias linaria Cav. Leaves. Molecules 2020, 25, 54.
- Bodoira, R.; Maestri, D. Phenolic compounds from nuts: Extraction, chemical profiles, and bioactivity. J. Agric. Food Chem. 2020, 68, 927–942.
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 26, 75–86.
- Servili, M.; Sordini, B.; Esposto, S.; Urbani, S.; Veneziani, G.; Di Maio, I.; Selvaggini, R.; Taticchi, A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants 2014, 3, 1–23.
- Bouyahya, A.; Chamkhi, I.; Benali, T.; Guaouguaou, F.-E.; Balahbibe, A.; El Omari, N.; Taha, D.; Belmehdi, O.; Ghokhan, Z.; El Menyiy, N. Traditional use, phytochemistry, toxicology, and pharmacology of Origanum majorana L. J. Ethnoph. 2021, 265, 113318.
- Mamede, L.; Ledoux, A.; Jansen, O.; Frédérich, M. Natural phenolic compounds and derivatives as potential antimalarial agents. Planta Med. 2020, 86, 585–618.
- Mazumder, K.; Biswas, B.; Raja, I.M.; Fukase, K. A review of cytotoxic plants of the indian subcontinent and a broad-spectrum analysis of their bioactive compounds. Molecules 2020, 25, 1904.
- Mansoori, B.; Mohammadi, A.; Doustvandi, M.A.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic therapy for cancer: Role of natural products. Photodiag. Photodyn. Ther. 2019, 26, 395–404.
- Da Fonsêca, D.V.; da Silva Maia Bezerra, C., Jr.; Cardoso Lima, T.; Nóbrega de Almeida, R.; Pergentino de Sousa, D. Anticonvulsant essential oils and their relationship with oxidative stress in epilepsy. Biomolecules 2019, 9, 835.
- Tabassum, H.; Ahmad, A.; Ahmad, I.Z. Nigella sativa L. and its bioactive constituents as hepatoprotectant: A review. Curr. Pharm. Biotechnol. 2018, 19, 43–67.
- Tepe, B.; Cakir, A.; Tepe, A.S. Medicinal uses, phytochemistry, and pharmacology of Origanum onites (L.): A review. Chem. Biodiv. 2016, 13, 504–520.
- Yamada, M.; Ono, K.; Hamaguchi, T.; Noguchi-Shinohara, M. Natural phenolic compounds as therapeutic and preventive agents for cerebral amyloidosis. In Natural Compounds as Therapeutic Agents for Amyloidogenic Diseases; Advances in Experimental Medicine and Biology; Vassallo, N., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 863, pp. 79–94.
- Gutiérrez-Grijalva, E.P.; Ambriz-Pére, D.L.; Leyva-López, N.; Castillo-López, R.I.; Heredia, J.B. Review: Dietary phenolic compounds, health benefits and bioaccessibility. Archiv. Latinoam. Nutr. 2016, 66, 87–100.
- Kimura, H.; Ohtsuka, K.; Matsumoto, A.; Ougi, T.; Ishibashi, Y.; Yamano, K. High performance phenolic resin based on untreated natural herbaceous lignin. Polym. Polym. Compos. 2015, 23, 525–534.
- Campos, D.; Betalleluz, I.; Tauquino, R.; Chirinos, R.; Pedreschi, R. Nutritional and functional characterisation of Andean chicuru (Stangea rhizanta). Food Chem. 2009, 112, 63–70.
- Lombardo, L.; Grasso, F.; Lanciano, F.; Loria, S.; Monetti, E. Broad-Spectrum health protection of extra virgin olive oil compounds. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 57, pp. 41–77.
- Kaushik, P.; Andújar, I.; Vilanova, S.; Plazas, M.; Gramazio, P.; Herraiz, F.J.; Brar, N.S.; Prohens, J. Breeding vegetables with increased content in bioactive phenolic acids. Molecules 2015, 20, 18464–18481.
- Dall’Acqua, S.; Ak, G.; Sut, S.; Zengin, G.; Yıldıztugay, E.; Mahomoodally, M.F.; Sinan, K.I.; Lobine, D. Comprehensive bioactivity and chemical characterization of the endemic plant Scorzonera hieraciifolia Hayek extracts: A promising source of bioactive compounds. Food Res. Int. 2020, 137, 109371.
- Zengin, G.; Mahomoodally, M.F.; Rocchetti, G.; Lucini, L.; Sieniawska, E.; Świątek, Ł.; Rajtar, B.; Polz-Dacewicz, M.; Senkardes, I.; Aktumsek, A.; et al. Chemical characterization and bioactive properties of different extracts from Fibigia clypeata, an unexplored plant food. Foods 2020, 9, 705.
- Quílez, M.; Ferreres, F.; López-Miranda, S.; Salazar, E.; Jordán, M.J. Seed oil from mediterranean aromatic and medicinal plants of the lamiaceae family as a source of bioactive components with nutritional. Antioxidants 2020, 9, 510.
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of the phenolic profile of Castanea sativa mill. by-products and their antioxidant and antimicrobial activity against multiresistant bacteria. Antioxidants 2020, 9, 87.
- Golkar, P.; Moattar, F. Essential oil composition, bioactive compounds, and antioxidant activities in Iberis amara L. Nat. Prod. Commun. 2019, 14, 1–8.
- Sut, S.; Dall’Acqua, S.; Zengin, G.; Senkardes, I.; Bulut, G.; Cvetanović, A.; Stupar, A.; Mandić, A.; Picot-Allain, C.; Dogan, A.; et al. Influence of different extraction techniques on the chemical profile and biological properties of Anthemis cotula L.: Multifunctional aspects for potential pharmaceutical applications. J. Pharm. Biomed. Anal. 2019, 173, 75–85.
- Petrović, M.; Pastor, F.; Ðurović, S.; Veljović, S.; Gorjanović, S.; Sredojević, M.; Vukosavljević, P. Evaluation of novel green walnut liqueur as a source of antioxidants: Multi-method approach. J. Food. Sci. Technol. 2021, 58, 2160–2169.
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial activity of naturally occurring phenols and derivatives against biofilm and planktonic bacteria. Front. Chem. 2019, 7, 653.
- Caleja, C.; Finimundy, T.C.; Pereira, C.; Barros, L.; Calhelha, R.C.; Sokovic, M.; Ivanov, M.; Carvalho, A.M.; Rosa, E.; Ferreira, I.C.F.R. Challenges of traditional herbal teas: Plant infusions and their mixtures with bioactive properties. Food Funct. 2019, 10, 5939–5951.
- Andrade, C.; Ferreres, F.; Gomes, N.G.M.; Duangsrisai, S.; Srisombat, N.; Vajrodaya, S.; Pereira, D.M.; Gil-Izquierdo, A.; Andrade, P.B.; Valentão, P. Phenolic profiling and biological potential of ficus curtipes corner leaves and stem bark: 5-lipoxygenase inhibition and interference with NO levels in LPS-stimulated RAW264.7 macrophages. Biomolecules 2019, 9, 400.
- Garzon, A.G.; Drago, S.R. Free a-amino acids, g-Aminobutyric acid (GABA), phenolic compounds and their relationships with antioxidant properties of sorghum malted in different conditions. J. Food Sci. Technol. 2018, 55, 3188–3198.
- Quintero-Flórez, A.; Pereira-Caro, G.; Sánchez-Quezada, C.; Moreno-Rojas, J.M.; Gaforio, J.J.; Jimenez, A.; Beltran, G. Effect of olive cultivar on bioaccessibility and antioxidant activity of phenolic fraction of virgin olive oil. Eur. J. Nutr. 2018, 57, 1925–1946.
- Uzun, Y.; Dalar, A.; Konczak, I. Sempervivum davisii: Phytochemical composition, antioxidant and lipase-inhibitory activities. Pharm. Biol. 2017, 55, 532–540.
- Atiya, A.; Sinha, B.N.; Lal, U.R. Bioactive phenylpropanoid analogues from Piper betle L. var. birkoli leaves. Nat. Prod. Res. 2017, 31, 2604–2611.
- Freitas dos Santos, H.; Ferreira Campos, J.; Miranda dos Santos, C.; Perrella Balestieri, J.B.; Brentan Silva, D.; Carollo, C.A.; de Picoli Souza, K.; Estevinho, L.M.; dos Santos, E.L. Chemical profile and antioxidant, anti-inflammatory, antimutagenic and antimicrobial activities of geopropolis from the stingless bee Melipona orbignyi. Int. J. Mol. Sci. 2017, 18, 953.
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26.
- Guenes, M.E.; Şahin, S.; Demir, C.; Borum, E.; Tosunoğlu, A. Determination of phenolic compounds profile in chestnut and floral honeys and their antioxidant and antimicrobial activities. J. Food Biochem. 2016, 41, e12345.
- Herraiz, F.J.; Villano, D.; Plazas, M.; Vilanova, S.; Ferreres, F.; Prohens, J.; Moreno, D.A. Phenolic profile and biological activities of the pepino (Solanum muricatum) fruit and its wild relative S. caripense. Int. J. Mol. Sci. 2016, 17, 394.
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Tech. 2015, 52, 5790–5798.
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the encapsulation of natural products: The case of chitosan biopolymer as a matrix. Pharmaceutics 2020, 12, 669.
- Delogu, G.; Juliano, C.C.A.; Usai, M. Thymus catharinae camarda essential oil: β-cyclodextrin inclusion complexes, evaluation of antimicrobial activity. Nat. Prod. Res. 2015, 30, 2049–2057.
- Dall’Acqua, S.; Kumar, G.; Sinan, K.I. An insight into Cochlospermum planchonii extracts obtained by traditional and green extraction methods: Relation between chemical compositions and biological properties by multivariate analysis. Ind. Crops Prod. 2020, 147, 112226.
- Nazeam, J.A.; AL-Shareef, W.A.; Helmy, M.W.; El-Haddad, A.E. Bioassay-guided isolation of potential bioactive constituents from pomegranate agrifood by-product. Food Chem. 2020, 326, 126993.
- Borrás Linares, I.; Arráez-Romána, D.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comparison of different extraction procedures for the comprehensive characterization of bioactive phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight mass spectrometry. J. Chrom. A 2011, 1218, 7682–7690.
- Alves-Silva, J.M.; Guerra, I.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Figueirinha, A.; Salgueiro, L. Chemical composition of Crithmum maritimum L. essential oil and hydrodistillation residual water by GC-MS and HPLC-DAD-MS/MS, and their biological activities. Ind. Crops Prod. 2020, 149, 112329.
- Mahomoodally, M.F.; Dall’Acqua, S.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Etienne, O.K.; Sadeer, N.B.; Ak, G.; Zengin, G. Phenolic compounds analysis of three Euphorbia species by LC-DAD-MSn and their biological properties. J. Pharm. Biomed. Anal. 2020, 189, 113477.
- Ma, W.; Tang, B.; Row, K.H. Exploration of a ternary deep eutectic solvent of methyltriphenylphosphonium bromide/chalcone/formic acid for the selective recognition of rutin and quercetin in Herba Artemisiae scopariae. J. Sep. Sci. 2017, 40, 3248–3256.
- Arun, K.P.; Brindha, P. Investigations into phenolic and alkaloid constituents of Jatropha tanjorensis by LC-MS/MS and evaluating its bioactive property. Asian J. Chem. 2015, 27, 3249–3253.
- Oldoni, T.L.C.; Melo, P.S.; Massarioli, A.P.; Moreno, I.A.M.; Bezerra, R.M.N.; Rosalen, P.L.; da Silva, G.V.J.; Nascimento, A.M.; Alencar, S.M. Bioassay-guided isolation of proanthocyanidins with antioxidant activity from peanut (Arachis hypogaea) skin by combination of chromatography techniques. Food Chem. 2016, 192, 306–312.
- Alagawany, M.; Farag, M.R.; Salah, A.A.; Mahmoud, M.A. The role of oregano herb and its derivatives as immunomodulators in fish. Rev. Aquacult. 2020, 12, 2481–2492.
- Sorrenti, V.; Fortinguerra, S.; Caudullo, G.; Buriani, A. Deciphering the role of polyphenols in sports performance: From nutritional genomics to the gut microbiota toward phytonutritional epigenomics. Nutrients 2020, 12, 1265.
- Araghi, M.; Moslehi, Z.; Nafchi, A.A.; Mostahsan, A.; Salamat, N.; Garmakhany, A.D. Cold water fish gelatin modification by a natural phenolic cross-linker (ferulic acid and caffeic acid). Food Sci. Nutr. 2015, 3, 370–375.
- Zhang, X.; Do, M.D.; Casey, P.; Sulistio, A.; Qiao, G.G.; Lundin, L.; Lillford, P.; Kosaraju, S. Chemical modification of gelatin by a natural phenolic cross-linker, tannic acid. J. Agric. Food Chem. 2010, 58, 6809–6815.
- Alirezalua, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and technofunctional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306.
- Gullón, P.; Astray, G.; Gullón, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate peel as suitable source of high-added value bioactives: Tailored functionalized meat products. Molecules 2020, 25, 2859.
- Mainente, F.; Menin, A.; Alberton, A.; Zoccatelli, G.; Rizzi, C. Evaluation of the sensory and physical properties of meat and fish derivatives containing grape pomace powders International. J. Food Sci. Tech. 2018, 54, 952–958.
- Gutiérrez-del-Río, I.; Fernández, J.; Lombó, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicr. Agents 2018, 52, 309–315.
- Akl, E.M.; Dacrory, S.; Abdel-Aziz, M.S.; Kamel, S.; Fahim, A.M. Preparation and characterization of novel antibacterial blended films based on modified carboxymethyl cellulose/phenolic compounds. Polym. Bull. 2021, 78, 1061–1085.
- Ibrahim, S.A.; Soliman, O.R. Nano-encapsulation of bioactive oils through emulsion polymerization as antimicrobials and its adhesion to packaging films. Der Pharm. Lett. 2016, 8, 367–373.
- Jing, S.; Li, T.; Li, X.; Xu, Q.; Hu, J.; Li, R. Phenolic foams modified by cardanol through bisphenol modification. J. Appl. Polym. Sci. 2014, 131, 39942.
- Mahendran, A.R.; Wuzella, G.; Aust, N.; Mueller, U.; Kandelbauer, A. Processing and characterization of natural fibre reinforced composites using lignin phenolic binder. Polym. Polym. Comp. 2013, 21, 199–205.
- Rahim, M.A.; Kristufek, S.L.; Pan, S.; Richardson, J.J.; Caruso, F. Phenolic building blocks for the assembly of functional materials. Angew. Chem. Int. Ed. 2019, 58, 1904–1927.
- Lomonaco, D.; Maia, F.J.N.; Clemente, C.S.; Mota, J.P.F.; Costa, A.E., Jr.; Mazzetto, S.E. Thermal studies of new biodiesel antioxidants synthesized from a natural occurring phenolic lipid. Fuel 2012, 97, 552–559.
- Morales, J.C.; Lucas, R. Structure-activity relationship of phenolic antioxidants and olive components. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 905–914.
- Chen, Y.; Xiao, H.; Zheng, J.; Liang, G. Structure-thermodynamics-antioxidant activity relationships of selected natural phenolic acids and derivatives: An experimental and theoretical evaluation. PLoS ONE 2015, 10, e0121276.
- Zeljković, S.C.; Topčagic, A.; Požgan, F.; Štefane, B.; Tarkowski, P.; Maksimović, M. Antioxidant activity of natural and modified phenolic extracts from Satureja montana L. Ind. Crops Prod. 2015, 76, 1094–1099.
- Sun, Q.; Heilmann, J.; König, B. Natural phenolic metabolites with anti-angiogenic properties—A review from the chemical point of view. Beilstein J. Org. Chem. 2015, 11, 249–264.
- Groussin, A.-L.; Antoniotti, S. Valuable chemicals by the enzymatic modification of molecules of natural origin: Terpenoids, steroids, phenolics and related compounds. Biores. Technol. 2012, 115, 237–243.
- Cravens, A.; Payne, J.; Smolke, C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat. Commun. 2019, 10, 2142.
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and their derivatives—Recent development in biological and medical applications. Int. J. Mol. Sci. 2020, 21, 7078.
- Pawełczyk, A.; Olender, D.; Sowa-Kasprzak, K.; Zaprutko, L. Linked drug-drug conjugates based on triterpene and phenol structures. Rational synthesis, molecular properties, toxicity and bioactivity prediction. Arab. J. Chem. 2020, 13, 8793–8806.
- Nesterkina, M.; Kravchenko, I. Synthesis and pharmacological properties of novel esters based on monoterpenoids and glycine. Pharmaceuticals 2017, 10, 47.
- Rajput, J.D.; Bagul, S.D.; Pete, U.D.; Zade, C.M.; Padhye, S.B.; Bendre, R.S. Perspectives on medicinal properties of natural phenolic monoterpenoids and their hybrids. Mol. Divers. 2018, 22, 225–245.
- Mastelić, J.; Jerković, I.; Blažević, I.; Poljak-Blaži, M.; Borović, S.; Ivanćić-Baće, I.; Smrećki, V.; Žarković, N.; Brćić-Kostic, K.; Vikić-Topić, D. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J. Agric. Food Chem. 2008, 56, 3989–3996.
- Schreiner, L.; Bauer, J.; Ortner, E.; Buettner, A. Structure−Odor activity studies on derivatives of aromatic and oxygenated monoterpenoids synthesized by modifying p-cymene. J. Nat. Prod. 2020, 83, 834–842.
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001.
- Zang, H.; Shen, P.; Xu, Q.; Zhang, L.; Xia, G.; Sun, J.; Zhu, J.; Yang, X. Synthesis and biological activities of tyrosol phenolic acid ester derivatives. Chem. Nat. Compd. 2019, 55, 1043–1049.
- Barontini, M.; Bernini, R.; Carastro, I.; Gentili, P.; Romani, A. Synthesis and DPPH radical scavenging activity of novel compounds obtained from tyrosol and cinnamic acid derivatives. New J. Chem. 2014, 38, 809–816.
- Adelina Lombrea; Diana Antal; Florina Ardelean; Stefana Avram; Ioana Zinuca Pavel; Lavinia Vlaia; Ana-Maria Mut; Zorita Diaconeasa; Cristina Adriana Dehelean; Codruta Soica; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum Vulgare L. Essential Oil. International Journal of Molecular Sciences 2020, 21, 9653, 10.3390/ijms21249653.
- Mariam Aljaafari; Asma AlAli; Laila Baqais; Maream Alqubaisy; Mudhi AlAli; Aidin Molouki; Janna Ong-Abdullah; Aisha Abushelaibi; Kok-Song Lai; Swee-Hua Lim; et al. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules 2021, 26, 628, 10.3390/molecules26030628.
- Seyed Mohammad Nabavi; Anna Marchese; Morteza Izadi; Valeria Curti; Maria Daglia; Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chemistry 2015, 173, 339-347, 10.1016/j.foodchem.2014.10.042.
- Lisa Marinelli; Antonio DI Stefano; Ivana Cacciatore; Carvacrol and its derivatives as antibacterial agents. Phytochemistry Reviews 2018, 17, 903-921, 10.1007/s11101-018-9569-x.
- Bruna Mesquita; Patrícia Nascimento; Luciana Souza; Iolanda Farias; Romézio Silva; Telma Lemos; Francisco Monte; Irvila Oliveira; Maria Teresa Trevisan; Horlando Silva; et al. SYNTHESIS, LARVICIDAL AND ACETYLCHOLINESTERASE INHIBITORY ACTIVITIES OF CARVACROL/THYMOL AND DERIVATIVES. Química Nova 2018, 41, 412-416, 10.21577/0100-4042.20170189.
- Anna Marchese; Ilkay Erdogan Orhan; Maria Daglia; Ramona Barbieri; Arianna Di Lorenzo; Seyed Fazel Nabavi; Olga Gortzi; Morteza Izadi; Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chemistry 2016, 210, 402-414, 10.1016/j.foodchem.2016.04.111.
- Ajai Kumar; Suriya P. Singh; Sudarshan S. Chhokar; Thymol and its Derivatives as Antimicrobial Agents. Natural Product Communications 2008, 3, 823-828, 10.1177/1934578x0800300528.
- Armando Talavera-Alemán; Gabriela Rodríguez-García; Yliana López; Hugo A. García-Gutiérrez; J. Martín Torres-Valencia; Rosa E. del Río; Carlos M. Cerda-García-Rojas; Pedro Joseph-Nathan; Mario A. Gómez-Hurtado; Systematic evaluation of thymol derivatives possessing stereogenic or prostereogenic centers. Phytochemistry Reviews 2015, 15, 251-277, 10.1007/s11101-015-9412-6.
- Irene De Silvestro; Samuel L. Drew; Gary S. Nichol; Fernanda Duarte; Andrew L. Lawrence; Total Synthesis of a Dimeric Thymol Derivative Isolated from Arnica sachalinensis. Angewandte Chemie International Edition 2017, 56, 6813-6817, 10.1002/anie.201701481.
- Mostafa El-Miligy; Aly A. Hazzaa; Saad R. El-Zemity; Ahmed K. Al-Kubeisi; Synthesis of Thymol Derivatives as Potential Non-Irritant Antimicrobial and Insecticidal Agents. Current Bioactive Compounds 2019, 15, 125-137, 10.2174/1573407213666171115161626.
- Rafael A. Cherkasov; Ilyas S. Nizamov; Gulnara T. Gabdullina; Lyubov A. Almetkina; Radik R. Shamilov; Artem V. Sofronov; Dithiophosphoric and Dithiophosphonic Acids and Their Derivatives on the Basis of Thymol: Synthesis and Antimicrobial Activity. Phosphorus, Sulfur, and Silicon and the Related Elements 2013, 188, 33-35, 10.1080/10426507.2012.740697.
- Garima Matela; Robina Aman; Chetan Sharma; Smita Chaudhary; Reactions of tin- and triorganotin(IV) isopropoxides with thymol derivative: Synthesis, characterization and in vitro antimicrobial screening. Journal of the Serbian Chemical Society 2013, 78, 1323-1333, 10.2298/jsc120917030m.
- Sara Robledo; Edison Osorio; Diana Muñoz; Luz Marina Jaramillo; Adriana Restrepo; Gabriel Arango; Iván Vélez; In Vitro and In Vivo Cytotoxicities and Antileishmanial Activities of Thymol and Hemisynthetic Derivatives. Antimicrobial Agents and Chemotherapy 2005, 49, 1652-1655, 10.1128/aac.49.4.1652-1655.2005.
- D. H. More; N. S. Pawar; P. M. Dewang; S. L. Patil; P. P. Mahulikar; Microwave-assisted Sinthesis of Thymyl Ethers and Esters in Aqueous Medium. Russian Journal of General Chemistry 2004, 74, 217-218, 10.1023/b:rugc.0000025504.59745.09.
- Ranjeet Kaur; Mahendra P. Darokar; Sunil Kumar Chattopadhyay; Vinay Krishna; Ateeque Ahmad; Synthesis of halogenated derivatives of thymol and their antimicrobial activities. Medicinal Chemistry Research 2014, 23, 2212-2217, 10.1007/s00044-013-0809-8.
- Laura Getrey; Thomas Krieg; Frank Hollmann; Jens Schrader; Dirk Holtmann; Enzymatic halogenation of the phenolic monoterpenes thymol and carvacrol with chloroperoxidase. Green Chemistry 2014, 16, 1104-1108, 10.1039/c3gc42269k.
- Federica Sabuzi; Ekaterina Churakova; Pierluca Galloni; Ron Wever; Frank Hollmann; Barbara Floris; Valeria Conte; Thymol Bromination - A Comparison between Enzymatic and Chemical Catalysis. European Journal of Inorganic Chemistry 2015, 2015, 3519-3525, 10.1002/ejic.201500086.
- Barbara Floris; Federica Sabuzi; Alessia Coletti; Valeria Conte; Sustainable vanadium-catalyzed oxidation of organic substrates with H2O2. Catalysis Today 2017, 285, 49-56, 10.1016/j.cattod.2016.11.006.
- Federica Sabuzi; Giuseppe Pomarico; Barbara Floris; Francesca Valentini; Pierluca Galloni; Valeria Conte; Sustainable bromination of organic compounds: A critical review. Coordination Chemistry Reviews 2019, 385, 100-136, 10.1016/j.ccr.2019.01.013.
- Claudio Piombino; Heiko Lange; Federica Sabuzi; Pierluca Galloni; Valeria Conte; Claudia Crestini; Lignosulfonate Microcapsules for Delivery and Controlled Release of Thymol and Derivatives. Molecules 2020, 25, 866, 10.3390/molecules25040866.
- Magdalena Ulanowska; Beata Olas; Biological Properties and Prospects for the Application of Eugenol—A Review. International Journal of Molecular Sciences 2021, 22, 3671, 10.3390/ijms22073671.
- Tina Modjinou; Davy-Louis Versace; Samir Abbad-Andallousi; Noureddine Bousserrhine; Pierre Dubot; Valérie Langlois; Estelle Renard; Antibacterial and antioxidant bio-based networks derived from eugenol using photo-activated thiol-ene reaction. Reactive and Functional Polymers 2016, 101, 47-53, 10.1016/j.reactfunctpolym.2016.02.002.
- Francisco Felipe Maia Da Silva; Francisco José Queiroz Monte; Telma Leda Gomes De Lemos; Patrícia Georgina Garcia Do Nascimento; Alana Kelly De Medeiros Costa; Luanda Misley Mota De Paiva; Eugenol derivatives: synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chemistry Central Journal 2018, 12, 34, 10.1186/s13065-018-0407-4.
- Mamilla R. Charan Raja; Anand Babu Velappan; Davidraj Chellappan; Joy Debnath; Santanu Kar Mahapatra; Eugenol derived immunomodulatory molecules against visceral leishmaniasis. European Journal of Medicinal Chemistry 2017, 139, 503-518, 10.1016/j.ejmech.2017.08.030.
- Nurul Hazwani Che Abdul Rahim; Asnuzilawati Asari; Noraznawati Ismail; Hasnah Osman; Synthesis and Antibacterial Study of Eugenol Derivatives. Asian Journal of Chemistry 2017, 29, 22-26, 10.14233/ajchem.2017.20100.
- Somayeh Behrouz; Mohammad Navid Soltani Rad; Bahareh Taghavi Shahraki; Mohammad Fathalipour; Marzieh Behrouz; Hossein Mirkhani; Design, synthesis, and in silico studies of novel eugenyloxy propanol azole derivatives having potent antinociceptive activity and evaluation of their β-adrenoceptor blocking property. Molecular Diversity 2019, 23, 147-164, 10.1007/s11030-018-9867-7.
- Maria José G. Fernandes; Renato B. Pereira; David M. Pereira; A. Gil Fortes; Elisabete M. S. Castanheira; M. Sameiro T. Gonçalves; New Eugenol Derivatives with Enhanced Insecticidal Activity. International Journal of Molecular Sciences 2020, 21, 9257, 10.3390/ijms21239257.
- Eder João Lenardão; Raquel G. Jacob; Katiúcia D. Mesquita; Renata G. Lara; Rodrigo Webber; Débora Martins Martinez; Lucielli Savegnago; Samuel R. Mendes; Diego Alves; Gelson Perin; et al. Glycerol as a promoting and recyclable medium for catalyst-free synthesis of linear thioethers: new antioxidants from eugenol. Green Chemistry Letters and Reviews 2013, 6, 269-276, 10.1080/17518253.2013.811298.
- Héctor Carrasco; Marcela Raimondi; Laura Svetaz; Melina Di Liberto; María V. Rodriguez; Luis Espinoza; Alejandro Madrid; Susana Zacchino; Antifungal Activity of Eugenol Analogues. Influence of Different Substituents and Studies on Mechanism of Action. Molecules 2012, 17, 1002-1024, 10.3390/molecules17011002.
- Larissa Incerti Santos De Carvalho; Dalila Junqueira Alvarenga; Letícia Cruz Ferreira Do Carmo; Lucas Gomes De Oliveira; Naiara Chaves Silva; Amanda Latércia Tranches Dias; Luiz Felipe Leomil Coelho; Thiago Belarmino De Souza; Danielle Ferreira Dias; Diogo Teixeira Carvalho; et al. Antifungal Activity of New Eugenol-Benzoxazole Hybrids against Candida spp.. Journal of Chemistry 2017, 2017, 5207439 , 10.1155/2017/5207439.
- Andrés F. Olea; Angelica Bravo; Rolando Martínez; Mario Thomas; Claudia Sedan; Luis Espinoza; Elisabeth Zambrano; Denisse Carvajal; Evelyn Silva-Moreno; Héctor Carrasco; et al. Antifungal Activity of Eugenol Derivatives against Botrytis Cinerea. Molecules 2019, 24, 1239, 10.3390/molecules24071239.
- Waqas Nawaz; Zhongqin Zhou; Sa Deng; Xiaodong Ma; Xiaochi Ma; Chuangang Li; Xiaohong Shu; Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 1188, 10.3390/nu9111188.
- Paulina Pecyna; Joanna Wargula; Marek Murias; Malgorzata Kucinska; More than Resveratrol: New Insights into Stilbene-Based Compounds. Biomolecules 2020, 10, 1111, 10.3390/biom10081111.
- Pablo Peñalver; Efres Belmonte-Reche; Norma Adán; Marta Caro; Marisa Mateos Martin; Mario Delgado; Elena González-Rey; Juan Carlos Morales; Alkylated resveratrol prodrugs and metabolites as potential therapeutics for neurodegenerative diseases. European Journal of Medicinal Chemistry 2018, 146, 123-138, 10.1016/j.ejmech.2018.01.037.
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84.
- Khadem, S.; Marles, R.J. Monocyclic phenolic acids; hydroxy- and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005.
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513.
- Muronetz, V.I.; Barinova, K.; Kudryavtseva, S.; Medvedeva, M.; Melnikova, A.; Sevostyanova, I.; Semenyuk, P.; Stroylova, Y.; Sova, M. Natural and synthetic derivatives of hydroxycinnamic acid modulating the pathological transformation of amyloidogenic proteins. Molecules 2020, 25, 4647.
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Reports 2019, 24, e00370.
- Manuja, R.; Sachdeva, S.; Jain, A.; Chaudhary, J. A comprehensive review on biological activities of p-hydroxy benzoic acid and its derivatives. Int. J. Pharm. Sci. Rev. Res. 2013, 22, 109–115.
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic effects of simple phenolic acids: A comprehensive review. Phytother. Res. 2016, 30, 184–199.
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 26, 952943.
- Khan, A.K.; Rashid, R.; Nighat, F.; Sadaf, M.; Mir, S.; Khan, S.; Jabeen, N.; Murtaza, G. Pharmacological activities of protocatechuic acid. Acta Pol. Pharm. Drug Res. 2015, 72, 643–650.
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609.
- Naira, N.; Asdaq, S.M.B.; Heba, S.; Said, A.H.E.l.A. Gallic acid: A promising lead molecule for drug development. J. App. Pharm. 2016, 8, 213.
- Ou, S.; Kwok, K.-C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269.
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702.
- Brenelli de Paiva, L.; Goldbeck, R.; Dantas dos Santos, W.; Squina, F.M. Ferulic acid and derivatives: Molecules with potential application in the pharmaceutical field. Braz. J. Pharm. Sci. 2013, 49, 395–411.
- Pei, K.; Ou, J.; Huang, C.; Ou, S. Derivatives of ferulic acid: Structure, preparation and biological activities. Ann. Res. Rev. Biol. 2015, 5, 512–528.
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336.
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.C.W.; Woo, K.-S.; Fung, K.-P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246.
- See, as an example: Koufaki, M. Vitamin E derivatives: A patent review (2010–2015). Expert Opin. Ther. Pat. 2016, 26, 35–47.
- Kozubek, A.; Tyman, J.H.P. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem. Rev. 1999, 99, 1–25.
- Stasiuk, M.; Kozubek, A. Biological activity of phenolic lipids. Cell. Mol. Life Sci. 2010, 67, 841–860.
- Meshginfar, N.; Tavakoli, H.; Dornan, K.; Hosseinian, F.; Hosseinian, F. Phenolic lipids as unique bioactive compounds: A comprehensive review on their multifunctional activity toward the prevention of Alzheimer’s disease. Crit. Rev. Food Sci. Nutr. 2021, 61, 1394–1403.
- Teixeira dos Santos, A.; Coelho Bernardo Guerra, G.; Marques, J.I.; Torres-Rêgo, M.; Ferreira Alves, J.S.; Carvalho Vasconcelos, R.; Fernandes de Souza Araújo, D.; Silva Abreu, L.; Gomes de Carvalho, T.; Cavalcante de Araújo, D.R.; et al. Potentialities of cashew nut (Anacardium occidentale) by-product for pharmaceutical applications: Extraction and purification technologies, safety, and anti-inflammatory and anti-arthritis activities. Rev. Bras. Farm. 2020, 30, 652–666.
- Kashyap, D.; Kumar Garg, V.; Singh Tuli, H.; Yerer, M.B.; Sak, K.; Kumar Sharma, A.; Kumar, M.; Aggarwal, V.; Singh Sandhu, S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174.
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89.
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699.
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and its derivatives: Synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability. Anti-Canc. Ag. Med. Chem. 2009, 9, 138–161.
- Saladino, R.; Gualandi, G.; Farina, A.; Crestini, C.; Nencioni, L.; Palamara, A.T. Advances and challenges in the synthesis of highly oxidised natural phenols with antiviral, antioxidant and cytotoxic activities. Curr. Med. Chem. 2008, 15, 1500–1519.
- Dudnik, A.; Gaspar, P.; Neves, A.R.; Forster, J. Engineering of microbial cell factories for the production of plant polyphenols with health-beneficial properties. Curr. Pharm. Des. 2018, 24, 2208–2225.
- Preetha Anand; Sherin G. Thomas; Ajaikumar B. Kunnumakkara; Chitra Sundaram; Kuzhuvelil B. Harikumar; Bokyung Sung; Sheeja T. Tharakan; Krishna Misra; Indira K. Priyadarsini; Kallikat N. Rajasekharan; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochemical Pharmacology 2008, 76, 1590-1611, 10.1016/j.bcp.2008.08.008.
- Syed Nasir Abbas Bukhari; Ibrahim Jantan; Malina Jasamai; Waqas Ahmad; Muhammad Wahab Bin Amjad; Synthesis and Biological Evaluation of Curcumin Analogues. Journal of Medical Sciences 2013, 13, 501-513, 10.3923/jms.2013.501.513.
- Augustine Amalraj; Anitha Pius; Sreerag Gopi; Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives – A review. Journal of Traditional and Complementary Medicine 2016, 7, 205-233, 10.1016/j.jtcme.2016.05.005.
- Qiu Sun; Jörg Heilmann; Burkhard König; Natural phenolic metabolites with anti-angiogenic properties – a review from the chemical point of view. Beilstein Journal of Organic Chemistry 2015, 11, 249-264, 10.3762/bjoc.11.28.
- Tianpeng Chen; Gaoyang Zhu; Xiangwei Meng; Xingxian Zhang; Recent developments of small molecules with anti-inflammatory activities for the treatment of acute lung injury. European Journal of Medicinal Chemistry 2020, 207, 112660, 10.1016/j.ejmech.2020.112660.
- Fiona C. Rodrigues; Nv Anil Kumar; Goutam Thakur; Developments in the anticancer activity of structurally modified curcumin: An up-to-date review. European Journal of Medicinal Chemistry 2019, 177, 76-104, 10.1016/j.ejmech.2019.04.058.
- Sawsan Noureddin; Reda M. El-Shishtawy; Khalid O. Al-Footy; Curcumin analogues and their hybrid molecules as multifunctional drugs. European Journal of Medicinal Chemistry 2019, 182, 111631, 10.1016/j.ejmech.2019.111631.
- Zintle Mbese; Vuyolwethu Khwaza; Blessing Atim Aderibigbe; Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules 2019, 24, 4386, 10.3390/molecules24234386.
- Mahtab Zangui; Stephen L. Atkin; Muhammed Majeed; Amirhossein Sahebkar; Current evidence and future perspectives for curcumin and its analogues as promising adjuncts to oxaliplatin: state-of-the-art. Pharmacological Research 2019, 141, 343-356, 10.1016/j.phrs.2019.01.020.
- Filippa Lo Cascio; Paola Marzullo; Rakez Kayed; Antonio Palumbo Piccionello; Curcumin as Scaffold for Drug Discovery against Neurodegenerative Diseases. Biomedicines 2021, 9, 173, 10.3390/biomedicines9020173.
- Eirini Chainoglou; Dimitra Hadjipavlou-Litina; Curcumin in Health and Diseases: Alzheimer’s Disease and Curcumin Analogues, Derivatives, and Hybrids. International Journal of Molecular Sciences 2020, 21, 1975, 10.3390/ijms21061975.
- Kamran Mansouri; Shna Rasoulpoor; Alireza Daneshkhah; Soroush Abolfathi; Nader Salari; Masoud Mohammadi; Shervin Shabani; Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791, 10.1186/s12885-020-07256-8.
