Natural killer T (NKT) cells, a small population of T cells, are capable of influencing a wide range of the immune cells, including T cells, B cells, dendritic cells and macrophages. NKT cells can influence the status of the innate and adaptive immune systems because they secrete huge amounts of Th1 and Th2 cytokines. Earlier, the NKT cells were characterized by the NK and T cell properties as they express the natural killer (NK) cell lineage markers and αβ T-cell receptor (TCR). NKT cells are more appropriately defined as “CD1d-restricted and TCR-αβ positive T cells”. In mice, the NKT cells constitute about 0.2–2.0% of lymphocytes in the blood, spleen, bone marrow and thymus, and about 15–35% of total lymphocytes in the liver. On the other hand, the levels of NKT cells are lower in humans, comprising about 0.04–1.3% of circulating lymphocytes in the blood, spleen and bone marrow. They make up about 0.001–0.01% of lymphocytes in the thymus and about 1% in the liver. The greater part of the NKT cells, called canonical or invariant NKT cells (iNKT cells) or type I NKT cells have a specific TCR α-chain rearrangement (Vα14-Jα18 in mice; Vα24-Jα18 in humans), associated with limited diverse Vβ chains. Type II NKT cells, also called non-classical NKT cells, are more diverse in TCR α-chain (but some Vα3.2-Jα9, Vα8 in mice) and TCR-β chains (but some Vβ8.2 in mice).
- NKT cells
- vaccine
- lipid ligands
1. Introduction
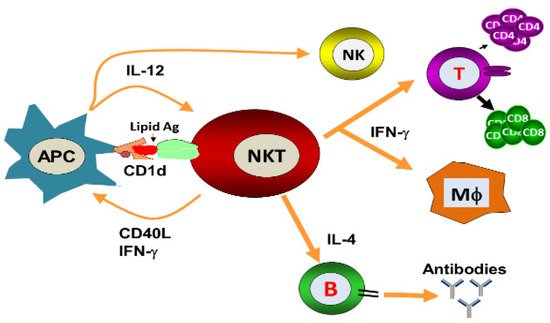
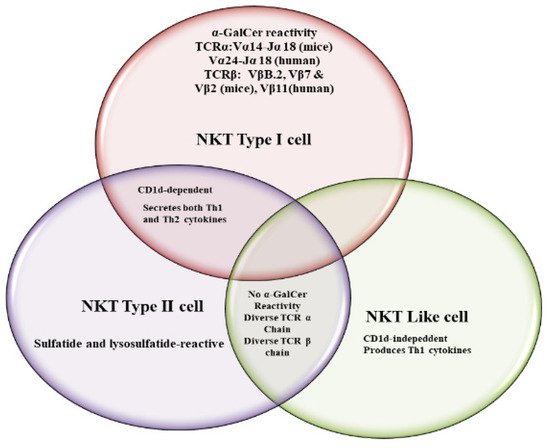
2. NKT Cell Ligands
3. Role of iNKT Cells against Viral Infections
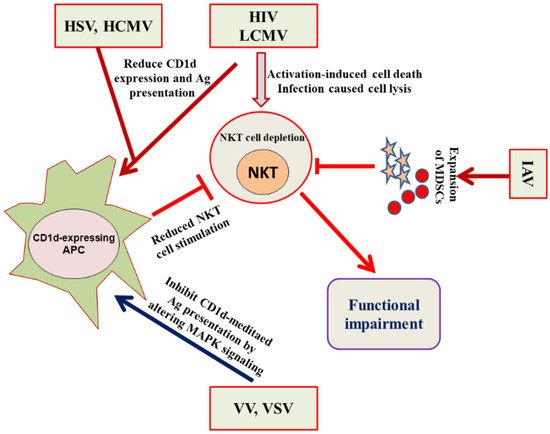
4. Evasion of the NKT Cell Functioning by Viruses
This entry is adapted from the peer-reviewed paper 10.3390/vaccines9090949
References
- Bendelac, A.; Savage, P.B.; Teyton, L. The Biology of NKT Cells. Annu. Rev. Immunol. 2007, 25, 297–336.
- Godfrey, D.I.; Stankovic, S.; Baxter, A. Raising the NKT cell family. Nat. Immunol. 2010, 11, 197–206.
- Berzins, S.P.; Smyth, M.; Baxter, A. Presumed guilty: Natural killer T cell defects and human disease. Nat. Rev. Immunol. 2011, 11, 131–142.
- Morita, M.; Motoki, K.; Akimoto, K.; Natori, T.; Sakai, T.; Sawa, E.; Yamaji, K.; Koezuka, Y.; Kobayashi, E.; Fukushima, H. Structure-Activity relationship of α-Galactosylceramide against B-16 bearing mice. J. Med. Chem. 1995, 38, 2176–2187.
- Brown, L.C.W.; Penaranda, C.; Kashyap, P.C.; Williams, B.B.; Clardy, J.; Kronenberg, M.; Sonnenburg, J.L.; Comstock, L.E.; Bluestone, J.A.; Fischbach, M.A. Production of α-Galactosylceramide by a Prominent Member of the Human Gut Microbiota. PLoS Biol. 2013, 11, e1001610.
- von Gerichten, J.; Schlosser, K.; Lamprecht, D.; Morace, I.; Eckhardt, M.; Wachten, D.; Jennemann, R.; Gröne, H.-J.; Mack, M.; Sandhoff, R. Diastereomer-specific quantification of bioactive hexosylceramides from bacteria and mammals. J. Lipid Res. 2017, 58, 1247–1258.
- Sriram, V.; Du, W.; Gervay-Hague, J.; Brutkiewicz, R.R. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur. J. Immunol. 2005, 35, 1692–1701.
- Brutkiewicz, R.R. CD1d ligands: The good, the bad, and the ugly. J. Immunol. 2006, 177, 769–775.
- Albacker, L.A.; Chaudhary, V.; Chang, Y.J.; Kim, H.Y.; Chuang, Y.T.; Pichavant, M.; DeKruyff, R.H.; Savage, P.B.; Umetsu, D.T. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyper reactivity. Nat. Med. 2013, 19, 1297–1304.
- Dhodapkar, M.V.; Kumar, V. Type II NKT Cells and Their Emerging Role in Health and Disease. J. Immunol. 2017, 198, 1015–1021.
- Brutkiewicz, R.R.; Lin, Y.; Cho, S.; Hwang, Y.K.; Sriram, V.; Roberts, T.J. CD1d-mediated antigen presentation to natural killer T (NKT) cells. Crit. Rev. Immunol. 2003, 23, 403–419.
- Coquet, J.; Chakravarti, S.; Kyparissoudis, K.; McNab, F.W.; Pitt, L.A.; McKenzie, B.S.; Berzins, S.P.; Smyth, M.; Godfrey, D.I. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl. Acad. Sci. USA 2008, 105, 11287–11292.
- James, E.E.; Andrew, J.K.; Webb, T.J. Raising the roof: The preferential pharmacological stimulation of Th1 and Th2 responses mediated by NKT cells. Med. Res. Rev. 2014, 34, 45–76.
- Liu, Z.; Guo, J. NKT-Cell glycolipid agonist as adjuvant in synthetic vaccine. Carbohydr. Res. 2017, 452, 78–90.
- Kakimi, K.; Guidotti, L.G.; Koezuka, Y.; Chisari, F. Natural Killer T Cell Activation Inhibits Hepatitis B Virus Replication in Vivo. J. Exp. Med. 2000, 192, 921–930.
- Liu, J.; Glosson, N.L.; Du, W.; Gervay-Hague, J.; Brutkiewicz, R.R. A Thr/Ser dual residue motif in the cytoplasmic tail of human CD1d is important for the down-regulation of antigen presentation following a herpes simplex virus 1 infection. Immunology 2013, 140, 191–201.
- Exley, M.A.; Bigley, N.J.; Cheng, O.; Tahir, S.M.; Smiley, S.T.; Carter, Q.L.; Stills, H.F.; Grusby, M.J.; Koezuka, Y.; Taniguchi, M.; et al. CD1d-Reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis viruses. J. Leukoc. Biol. 2001, 69, 713–718.
- Diana, J.; Griseri, T.; Lagaye, S.; Beaudoin, L.; Autrusseau, E.; Gautron, A.-S.; Tomkiewicz, C.; Herbelin, A.; Barouki, R.; von Herrath, M.; et al. NKT Cell-Plasmacytoid Dendritic Cell Cooperation via OX40 Controls Viral Infection in a Tissue-Specific Manner. Immunity 2009, 30, 289–299.
- Ho, L.; Denney, L.; Luhn, K.; Teoh, D.; Clelland, C.; McMichael, A.J. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur. J. Immunol. 2008, 38, 1913–1922.
- van Dommelen, S.L.H.; Tabarias, H.A.; Smyth, M.J.; Degli-Esposti, M.A.; Law, L.M.J.; Everitt, J.C.; Beatch, M.D.; Holmes, C.F.B.; Hobman, T.C. Activation of Natural Killer (NK) T Cells during Murine Cytomegalovirus Infection Enhances the Antiviral Response Mediated by NK Cells. J. Virol. 2003, 77, 1764–1771.
- Wu, C.Y.; Feng, Y.; Qian, G.C.; Wu, J.H.; Luo, J.; Wang, Y.; Chen, G.J.; Guo, X.-K.; Wang, Z.J. α-Galactosylceramide protects mice from lethal Coxsackievirus B3 infection and subsequent myocarditis. Clin. Exp. Immunol. 2010, 162, 178–187.
- Johnson, T.R.; Hong, S.; Van Kaer, L.; Koezuka, Y.; Graham, B.S. NK T Cells Contribute to Expansion of CD8 + T Cells and Amplification of Antiviral Immune Responses to Respiratory Syncytial Virus. J. Virol. 2002, 76, 4294–4303.
- De Santo, C.; Salio, M.; Masri, S.H.; Lee, L.Y.-H.; Dong, T.; Speak, A.; Porubsky, S.; Booth, S.; Veerapen, N.; Besra, G.; et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus–induced myeloid-derived suppressor cells in mice and humans. J. Clin. Investig. 2008, 118, 4036–4048.
- Singh, D.; Ghate, M.; Godbole, S.; Kulkarni, S.; Thakar, M. Functional Invariant Natural Killer T Cells Secreting Cytokines Are Associated with Non-Progressive Human Immunodeficiency Virus-1 Infection but Not With Suppressive Anti-Retroviral Treatment. Front. Immunol. 2018, 9, 1152.
- Nichols, K.E.; Hom, J.; Gong, S.; Ganguly, A.; Ma, C.; Cannons, J.L.; Tangye, P.S.; Schwartzberg, P.L.; Koretzky, G.A.; Stein, P.L. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat. Med. 2005, 11, 340–345.
- Rigaud, S.; Fondanèche, M.-C.; Lambert, N.C.; Pasquier, B.; Mateo, V.; Soulas-Sprauel, P.; Galicier, L.; Le Deist, F.; Rieux-Laucat, F.; Revy, P.; et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature 2006, 444, 110–114.
- Locci, M.; Draghici, E.; Marangoni, F.; Bosticardo, M.; Catucci, M.; Aiuti, A.; Cancrini, C.; Marodi, L.; Espanol, T.; Bredius, R.G.; et al. The Wiskott-Aldrich syndrome protein is required for iNKT cell maturation and function. J. Exp. Med. 2009, 206, 735–742.
- Levy, O.; Orange, J.S.; Hibberd, P.; Steinberg, S.; LaRussa, P.; Weinberg, A.; Wilson, S.B.; Shaulov, A.; Fleisher, G.; Geha, R.S.; et al. Disseminated varicella infection due to the vaccine strain of varicella-zoster virus, in a patient with a novel deficiency in natural killer T cells. J. Infect. Dis. 2003, 188, 948–953.
- Grubor-Bauk, B.; Simmons, A.; Mayrhofer, G.; Speck, P.G. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J. Immunol. 2003, 170, 1430–1434.
- Yuan, W.; Dasgupta, A.; Cresswell, P. Herpes simplex virus evades naturalkiller T cell recognition by suppressing CD1d recycling. Nat. Immunol. 2006, 7, 835–842.
- Tyznik, A.J.; Tupin, E.; Nagarajan, N.A.; Her, M.J.; Benedict, C.A.; Kronenberg, M. Cutting edge: The mechanism of invariant NKT cell responses to viral danger signals. J. Immunol. 2008, 181, 4452–4456.
- Broxmeyer, H.E.; Dent, A.; Cooper, S.; Hangoc, G.; Wang, Z.-Y.; Du, W.; Gervay-Haque, J.; Sriram, V.; Renukaradhya, G.J.; Brutkiewicz, R.R. A role for natural killer T cells and CD1d molecules in counteracting suppression of hematopoiesis in mice induced by infection with murine cytomegalovirus. Exp. Hematol. 2007, 35, 87–93.
- Paget, C.; Ivanov, S.; Fontaine, J.; Blanc, F.; Pichavant, M.; Renneson, J.; Bialecki, E.; Pothlichet, J.; Vendeville, C.; Barba-Speath, G.; et al. Potential Role of Invariant NKT Cells in the Control of Pulmonary Inflammation and CD8+ T Cell Response during Acute Influenza A Virus H3N2 Pneumonia. J. Immunol. 2011, 186, 5590–5602.
- Ajuebor, M.N. Role of NKT cells in the digestive system. I. Invariant NKT cells and liver diseases: Is there strength in numbers? Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G651–G656.
- Wang, X.F.; Lei, Y.; Chen, M.; Chen, C.B.; Ren, H.; Shi, T.D. PD-1/PDL1 and CD28/CD80 pathways modulate naturalkiller T cell function to inhibit hepatitis B virus replication. J. Viral. Hepat. 2013, 20, 27–39.
- Zeissig, S.; Murata, K.; Sweet, L.; Publicover, J.; Hu, Z.; Kaser, A.; Bosse, E.; Iqbal, J.; Hussain, M.M.; Balschun, K.; et al. Hepatitis B virus-induced lipid alterations contribute to naturalkiller T cell-dependent protective immunity. Nat. Med. 2012, 18, 1060–1068.
- Villanueva, A.I.; Haeryfar, S.M.; Mallard, B.A.; Kulkarni, R.R.; Sharif, S. Functions of NKT cells are modulated by TLR ligands and IFNα. Innate Immun. 2015, 21, 275–288.
- Bowie, A.G.; Haga, I.R. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 2005, 42, 859–867.
- Schäfer, A.; Hühr, J.; Schwaiger, T.; Dorhoi, A.; Mettenleiter, T.C.; Blome, S.; Schröder, C.; Blohm, U. Porcine Invariant Natural Killer T Cells: Functional Profiling and Dynamics in Steady State and Viral Infections. Front. Immunol. 2019, 10, 1380.
- Renukaradhya, G.J.; Manickam, C.; Khatri, M.; Rauf, A.; Li, X.; Tsuji, M.; Rajashekara, G.; Dwivedi, V. Functional invariant NKT cells in pig lungs regulate the airway hyperreactivity: A potential animal model. J. Clin. Immunol. 2010, 31, 228–239.
- Artiaga, B.L.; Yang, G.; Hutchinson, T.E.; Loeb, J.C.; Richt, J.A.; Lednicky, J.A.; Salek-Ardakani, S.; Driver, J.P. Rapid control of pandemic H1N1 influenza by targeting NKT-cells. Sci. Rep. 2016, 6, 37999.
- Dwivedi, V.; Manickam, C.; Dhakal, S.; Binjawadagi, B.; Ouyang, K.; Hiremath, J.; Khatri, M.; Hague, J.G.; Lee, C.W.; Renukaradhya, G.J. Adjuvant effects of invariant NKT cell ligand potentiates the innate and adaptive immunity to an inactivated H1N1 swine influenza virus vaccine in pigs. Veter Microbiol. 2016, 186, 157–163.
- Pegu, A.; Asokan, M.; Wu, L.; Wang, K.; Hataye, J.; Casazza, J.P.; Guo, X.; Shi, W.; Georgiev, I.; Zhou, T.; et al. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat. Commun. 2015, 6, 8447.
- Courtney, A.N.; Nehete, P.; Nehete, B.P.; Thapa, P.; Zhou, D.; Sastry, K.J. Alpha-galactosylceramide is an effective mucosal adjuvant for repeated intranasal or oral delivery of HIV peptide antigens. Vaccine 2009, 27, 3335–3341.
- Sandberg, J.K.; Fast, N.M.; Palacios, E.H.; Fennelly, G.; Dobroszycki, J.; Palumbo, P.; Wiznia, A.; Grant, R.M.; Bhardwaj, N.; Rosenberg, M.G.; et al. Selective loss of innate CD4+ V alpha 24 natural killer T cells in human immunodeficiency virus infection. J. Virol. 2002, 76, 7528–7534.
- Moll, M.; Kuylenstierna, C.; Gonzalez, V.D.; Andersson, S.K.; Bosnjak, L.; Sönnerborg, A.; Quigley, M.F.; Sandberg, J.K. Severe functional impairment and elevated PD-1 expression in CD1d-restricted NKT cells retained during chronic HIV-1 infection. Eur. J. Immunol. 2009, 39, 902–911.
- Cho, S.; Knox, K.S.; Kohli, L.M.; He, J.J.; Exley, M.A.; Wilson, S.B.; Brutkiewicz, R.R. Impaired cell surface expression of human CD1d by the formation of an HIV-1 Nef/CD1d complex. Virology 2005, 337, 242–252.
- Chen, N.; McCarthy, C.; Drakesmith, H.; Li, D.; Cerundolo, V.; McMichael, A.J.; Screaton, G.R.; Xu, X.-N. HIV-1 down-regulates the expression of CD1d via Nef. Eur. J. Immunol. 2006, 36, 278–286.
- Motsinger-Reif, A.; Haas, D.W.; Stanic-Kostic, A.; Van Kaer, L.; Joyce, S.; Unutmaz, D. CD1d-restricted Human Natural Killer T Cells Are Highly Susceptible to Human Immunodeficiency Virus 1 Infection. J. Exp. Med. 2002, 195, 869–879.
- Fernandez, C.S.; Kelleher, A.D.; Finlayson, R.; Godfrey, D.I.; Kent, S.J. NKT cell depletion in humans during early HIV infection. Immunol. Cell Biol. 2014, 92, 578–590.
- Mureithi, M.W.; Cohen, K.; Moodley, R.; Poole, D.; Mncube, Z.; Kasmar, A.; Moody, D.B.; Goulder, P.J.; Walker, B.D.; Altfeld, M.; et al. Impairment of CD1d-Restricted Natural Killer T Cells in Chronic HIV Type 1 Clade C Infection. AIDS Res. Hum. Retroviruses 2011, 27, 501–509.
- Brutkiewicz, R.R. Cell Signaling Pathways That Regulate Antigen Presentation. J. Immunol. 2016, 197, 2971–2979.
- Renukaradhya, G.J.; Webb, T.; Khan, M.A.; Lin, Y.L.; Du, W.; Gervay-Hague, J.; Brutkiewicz, R. Virus-Induced Inhibition of CD1d1-Mediated Antigen Presentation: Reciprocal Regulation by p38 and ERK. J. Immunol. 2005, 175, 4301–4308.
- Liu, J.; Gallo, R.M.; Khan, M.A.; Iyer, A.K.; Kratzke, I.M.; Brutkiewicz, R.R. JNK2 modulates the CD1d-Dependent and -Independent activation of iNKT cells. Eur. J. Immunol. 2018, 49, 255–265.
