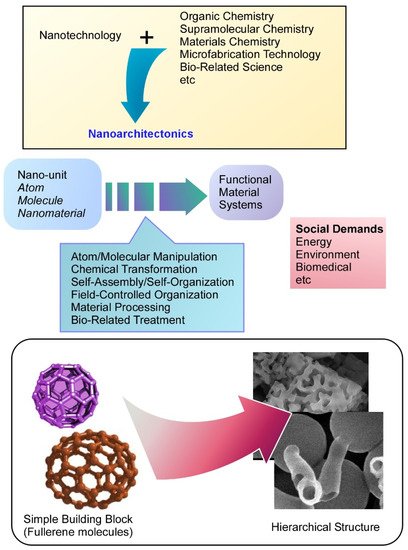
Fullerenes can be regarded as simple and fundamental building blocks with mono-elemental and zero-dimensional natures, these demonstrations for hierarchical functional structures impress the high capability of the nanoarchitectonics approaches. In fact, various hierarchical structures such as cubes with nanorods, hole-in-cube assemblies, face-selectively etched assemblies, and microstructures with mesoporous frameworks are fabricated by easy fabrication protocols. The fabricated fullerene assemblies have been used for various applications including volatile organic compound sensing, microparticle catching, supercapacitors, and photoluminescence systems.
- assembly
- fullerene
- hierarchical structure
- interface
- nanoarchitectonics
- nanomaterial
1. Introduction
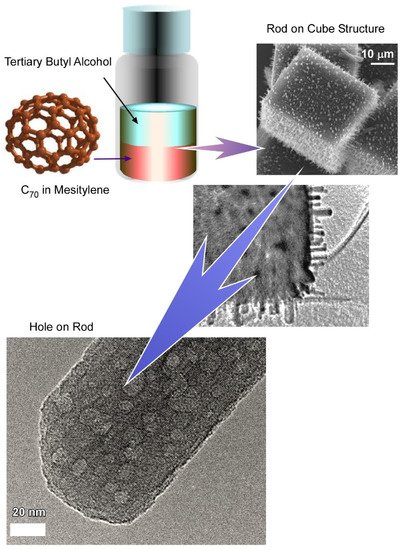
2. Hierarchically Structured Fullerene Assembly for Vapor Sensor Usage
2.1. Fullerene C70 Cube for Sensing Platform for Volatile Aromatic Solvent Vapor
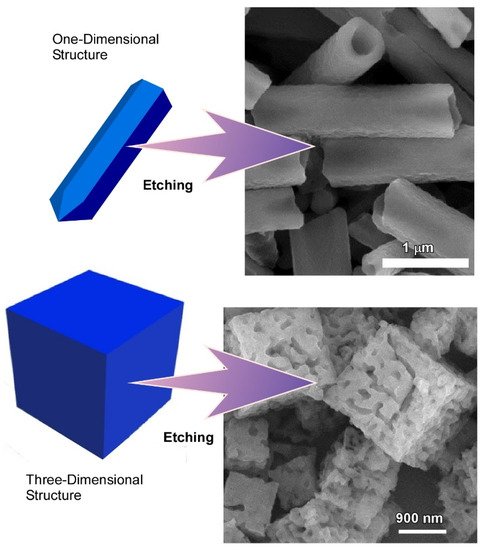
2.2. Dimension-Dependent Face-Selective Etching of Fullerene Assembly
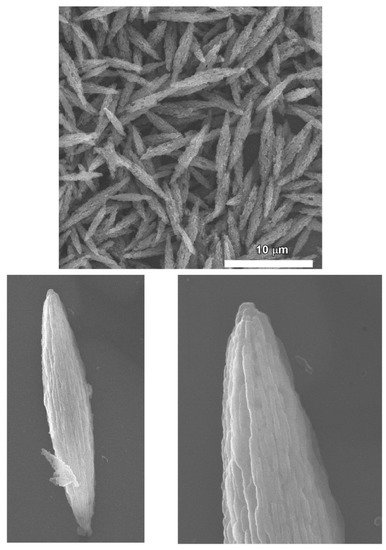
2.3. Bitter Melon Shaped Nanoporous Fullerene C60 Assembly
3. Fullerene Assembly with Microscopic Recognition Capability
3.1. Hole-in-Cube Fullerene Assembly with Microscopic Recognition Capability
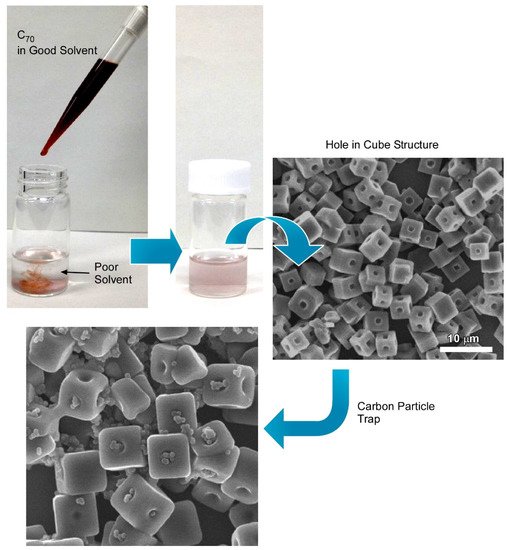
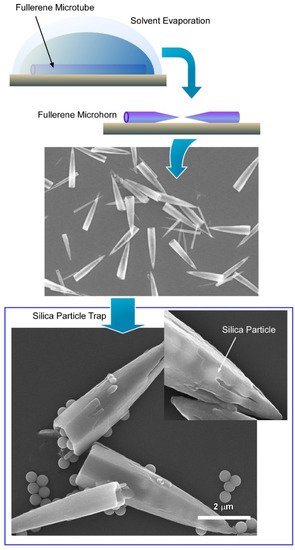
3.2. Fullerene Microhorns with Microscopic Recognition Properties
4. Fullerene Microstructure with Mesoporous Framework for Advanced Function
4.1. Mesoporous Fullerene C70 Cube with Enhanced Photoluminescence Property
4.2. Mesoporous Carbon Cubes for Supercapacitors
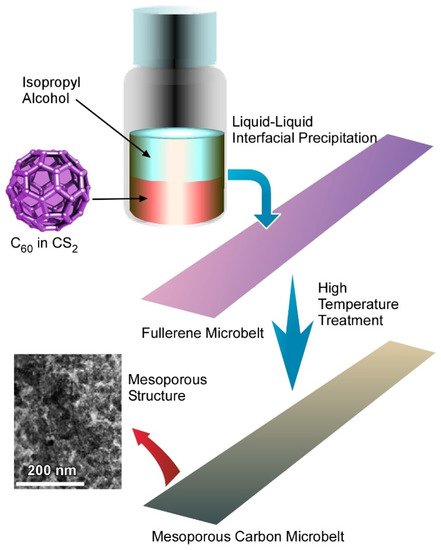
4.3. Quasi Two-Dimensional Mesoporous Carbon Microbelts for Supercapacitors
This entry is adapted from the peer-reviewed paper 10.3390/nano11082146
References
- Ariga, K. Nanoarchitectonics Revolution and Evolution: From Small Science to Big Technology. Small Sci. 2020, 1, 2000032.
- Ariga, K. Nanoarchitectonics at Interfaces for Regulations of Biorelated Phenomena: Small Structures with Big Effects. Small Struct. 2021, 2, 2100006.
- Ariga, K.; Li, J.; Fei, J.; Ji, Q.; Hill, J. Nanoarchitectonics for Dynamic Functional Materials from Atomic-/Molecular-Level Manipulation to Macroscopic Action. Adv. Mater. 2015, 28, 1251–1286.
- Sang, Y.; Liu, M. Nanoarchitectonics through supramolecular gelation: Formation and switching of diverse nanostructures. Mol. Syst. Des. Eng. 2018, 4, 11–28.
- Tirayaphanitchkul, C.; Imwiset, K.; Ogawa, M. Nanoarchitectonics through Organic Modification of Oxide Based Layered Materials; Concepts, Methods and Functions. Bull. Chem. Soc. Jpn. 2021, 94, 678–693.
- Liu, X.; Chen, T.; Gong, Y.; Li, C.; Niu, L.; Xu, S.; Xu, X.; Pan, L.; Shapter, J.G.; Yamauchi, Y.; et al. Light-conversion phosphor nanoarchitectonics for improved light harvesting in sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100404.
- Ramanathan, M.; Shrestha, L.; Mori, T.; Ji, Q.; Hill, J.; Ariga, K. Amphiphile nanoarchitectonics: From basic physical chemistry to advanced applications. Phys. Chem. Chem. Phys. 2013, 15, 10580–10611.
- Ariga, K.; Mori, T.; Kitao, T.; Uemura, T. Supramolecular chiral nanoarchitectonics. Adv. Mater. 2020, 32, 1905657.
- Cheng, P.; Wang, C.; Kaneti, Y.V.; Eguchi, M.; Lin, J.; Yamauchi, Y.; Na, J. Practical MOF Nanoarchitectonics: New Strategies for Enhancing the Processability of MOFs for Practical Applications. Langmuir 2020, 36, 4231–4249.
- Abe, H.; Liu, J.; Ariga, K. Catalytic nanoarchitectonics for environmentally compatible energy generation. Mater. Today 2015, 19, 12–18.
- Kumari, N.; Kumar, A.; Krishnan, V. Ultrathin Au–Ag Heterojunctions on Nanoarchitectonics Based Biomimetic Substrates for Dip Catalysis. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1954–1966.
- Chen, G.; Sciortino, F.; Ariga, K. Atomic Nanoarchitectonics for Catalysis. Adv. Mater. Interfaces 2020, 8, 2001395.
- Ishihara, S.; Labuta, J.; Van Rossom, W.; Ishikawa, D.; Minami, K.; Hill, J.; Ariga, K. Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys. Chem. Chem. Phys. 2014, 16, 9713–9746.
- Pandeeswar, M.; Senanayak, S.P.; Govindaraju, T. Nanoarchitectonics of Small Molecule and DNA for Ultrasensitive Detection of Mercury. ACS Appl. Mater. Interfaces 2016, 8, 30362–30371.
- Liu, J.; Zhou, H.; Yang, W.; Ariga, K. Soft Nanoarchitectonics for Enantioselective Biosensing. Accounts Chem. Res. 2020, 53, 644–653.
- Ariga, K.; Ji, Q.; Mori, T.; Naito, M.; Yamauchi, Y.; Abe, H.; Hill, J. Enzyme nanoarchitectonics: Organization and device application. Chem. Soc. Rev. 2013, 42, 6322–6345.
- Ariga, K.; Ito, M.; Mori, T.; Watanabe, S.; Takeya, J. Atom/molecular nanoarchitectonics for devices and related applications. Nano Today 2019, 28, 100762.
- Giussi, J.M.; Cortez, M.L.; Marmisollé, W.A.; Azzaroni, O. Practical use of polymer brushes in sustainable energy applications: Interfacial nanoarchitectonics for high-efficiency devices. Chem. Soc. Rev. 2019, 48, 814–849.
- Ariga, K.; Ishihara, S.; Abe, H.; Li, M.; Hill, J. Materials nanoarchitectonics for environmental remediation and sensing. J. Mater. Chem. 2011, 22, 2369–2377.
- Pham, T.; Qamar, A.; Dinh, T.; Masud, M.K.; Rais-Zadeh, M.; Senesky, D.G.; Yamauchi, Y.; Nguyen, N.; Phan, H. Nanoarchitectonics for Wide Bandgap Semiconductor Nanowires: Toward the Next Generation of Nanoelectromechanical Systems for Environmental Monitoring. Adv. Sci. 2020, 7, 2001294.
- Ariga, K. Nanoarchitectonics Can Save Our Planet: Nanoarchitectonics for Energy and Environment. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2243–2244.
- Kim, J.; Kim, J.H.; Ariga, K. Redox-active polymers for energy storage nanoarchitectonics. Joule 2017, 1, 739–768.
- Xu, J.; Zhang, J.; Zhang, W.; Lee, C.-S. Interlayer Nanoarchitectonics of Two-Dimensional Transition-Metal Dichalcogenides Nanosheets for Energy Storage and Conversion Applications. Adv. Energy Mater. 2017, 7, 1700571.
- Huang, H.; Yan, M.; Yang, C.; He, H.; Jiang, Q.; Yang, L.; Lu, Z.; Sun, Z.; Xu, X.; Bando, Y.; et al. Graphene Nanoarchitectonics: Recent Advances in Graphene-Based Electrocatalysts for Hydrogen Evolution Reaction. Adv. Mater. 2019, 31, e1903415.
- Stulz, E. Nanoarchitectonics with Porphyrin Functionalized DNA. Accounts Chem. Res. 2017, 50, 823–831.
- Liang, X.; Li, L.; Tang, J.; Komiyama, M.; Ariga, K. Dynamism of Supramolecular DNA/RNA Nanoarchitectonics: From Interlocked Structures to Molecular Machines. Bull. Chem. Soc. Jpn. 2020, 93, 581–603.
- Ariga, K.; Fakhrullin, R. Nanoarchitectonics on living cells. RSC Adv. 2021, 11, 18898–18914.
- Ariga, K.; Leong, D.T.; Mori, T. Nanoarchitectonics for Hybrid and Related Materials for Bio-Oriented Applications. Adv. Funct. Mater. 2017, 28, 1702905.
- Zhao, L.; Zou, Q.; Yan, X. Self-Assembling Peptide-Based Nanoarchitectonics. Bull. Chem. Soc. Jpn. 2019, 92, 70–79.
- Ariga, K.; Tsai, K.-C.; Shrestha, L.K.; Hsu, S.-H. Life science nanoarchitectonics at interfaces. Mater. Chem. Front. 2020, 5, 1018–1032.
- Ariga, K. There’s still plenty of room at the bottom. Chem. World 2021, 18, 5.
- Ariga, K.; Yamauchi, Y. Nanoarchitectonics from Atom to Life. Chem. Asian J. 2020, 15, 718–728.
- Aono, M.; Ariga, K. The Way to Nanoarchitectonics and the Way of Nanoarchitectonics. Adv. Mater. 2015, 28, 989–992.
- Ariga, K. Nanoarchitectonics: A navigator from materials to life. Mater. Chem. Front. 2016, 1, 208–211.
- Ariga, K.; Jia, X.; Song, J.; Hill, J.P.; Leong, D.T.; Jia, Y.; Li, J. Nanoarchitectonics beyond Self-Assembly: Challenges to Create Bio-Like Hierarchic Organization. Angew. Chem. Int. Ed. 2020, 59, 15424–15446.
- Bairi, P.; Minami, K.; Nakanishi, W.; Hill, J.; Ariga, K.; Shrestha, L. Hierarchically Structured Fullerene C70 Cube for Sensing Volatile Aromatic Solvent Vapors. ACS Nano 2016, 10, 6631–6637.
- Hsieh, C.-T.; Hsu, S.-H.; Maji, S.; Chahal, M.K.K.; Song, J.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Post-assembly dimension-dependent face-selective etching of fullerene crystals. Mater. Horiz. 2019, 7, 787–795.
- Furuuchi, N.; Shrestha, R.G.; Yamashita, Y.; Hirao, T.; Ariga, K.; Shrestha, L.K. Self-Assembled Fullerene Crystals as Excellent Aromatic Vapor Sensors. Sensors 2019, 19, 267.
- Bairi, P.; Minami, K.; Hill, J.P.; Ariga, K.; Shrestha, L.K. Intentional Closing/Opening of “Hole-in-Cube” Fullerene Crystals with Microscopic Recognition Properties. ACS Nano 2017, 11, 7790–7796.
- Tang, Q.; Maji, S.; Jiang, B.; Sun, J.; Zhao, W.; Hill, J.P.; Ariga, K.; Fuchs, H.; Ji, Q.; Shrestha, L.K. Manipulating the Structural Transformation of Fullerene Microtubes to Fullerene Microhorns Having Microscopic Recognition Properties. ACS Nano 2019, 13, 14005–14012.
- Bairi, P.; Tsuruoka, T.; Acharya, S.; Ji, Q.; Hill, J.P.; Ariga, K.; Yamauchi, Y.; Shrestha, L. Mesoporous fullerene C70 cubes with highly crystalline frameworks and unusually enhanced photoluminescence properties. Mater. Horiz. 2018, 5, 285–290.
- Wang, Q.; Zhang, Y.; Jiang, H.; Li, X.; Cheng, Y.; Meng, C. Designed mesoporous hollow sphere architecture metal (Mn, Co, Ni) silicate: A potential electrode material for flexible all solid-state asymmetric supercapacitor. Chem. Eng. J. 2019, 362, 818–829.
- Saito, Y.; Ashizawa, M.; Matsumoto, H. Mesoporous Hydrated Graphene Nanoribbon Electrodes for Efficient Supercapacitors: Effect of Nanoribbon Dispersion on Pore Structure. Bull. Chem. Soc. Jpn. 2020, 93, 1268–1274.
- Li, Y.; Henzie, J.; Park, T.; Wang, J.; Young, C.; Xie, H.; Yi, J.W.; Li, J.; Kim, M.; Kim, J.; et al. Fabrication of Flexible Microsupercapacitors with Binder-Free ZIF-8 Derived Carbon Films via Electrophoretic Deposition. Bull. Chem. Soc. Jpn. 2020, 93, 176–181.
- Nomura, K.; Nishihara, H.; Kobayashi, N.; Asada, T.; Kyotani, T. 4.4 V supercapacitors based on super-stable mesoporous carbon sheet made of edge-free graphene walls. Energy Environ. Sci. 2019, 12, 1542–1549.
- Kim, G.; Shiraki, T.; Fujigaya, T. Thermal Conversion of Triazine-Based Covalent Organic Frameworks to Nitrogen-Doped Nanoporous Carbons and Their Capacitor Performance. Bull. Chem. Soc. Jpn. 2020, 93, 414–420.
- Shrestha, R.L.; Chaudhary, R.; Shrestha, R.G.; Shrestha, T.; Maji, S.; Ariga, K.; Shrestha, L.K. Washnut Seed-Derived Ultrahigh Surface Area Nanoporous Carbons as High Rate Performance Electrode Material for Supercapacitors. Bull. Chem. Soc. Jpn. 2021, 94, 565–572.
- Shrestha, L.; Shrestha, R.G.; Yamauchi, Y.; Hill, J.; Nishimura, T.; Miyazawa, K.; Kawai, T.; Okada, S.; Wakabayashi, K.; Ariga, K. Nanoporous Carbon Tubes from Fullerene Crystals as the π-Electron Carbon Source. Angew. Chem. Int. Ed. 2014, 54, 951–955.
- Sengottaiyan, C.; Jayavel, R.; Shrestha, R.G.; Subramani, T.; Maji, S.; Kim, J.H.; Hill, J.P.; Ariga, K.; Shrestha, L. Indium Oxide/Carbon Nanotube/Reduced Graphene Oxide Ternary Nanocomposite with Enhanced Electrochemical Supercapacitance. Bull. Chem. Soc. Jpn. 2019, 92, 521–528.
- Baskar, A.V.; Ruban, A.M.; Davidraj, J.M.; Singh, G.; Al-Muhtaseb, A.H.; Lee, J.M.; Yi, J.; Vinu, A. Single-Step Synthesis of 2D Mesoporous C60/Carbon Hybrids for Supercapacitor and Li-Ion Battery Applications. Bull. Chem. Soc. Jpn. 2021, 94, 133–140.
- Bairi, P.; Maji, S.; Hill, J.P.; Kim, J.H.; Ariga, K.; Shrestha, L.K. Mesoporous carbon cubes derived from fullerene crystals as a high rate performance electrode material for supercapacitors. J. Mater. Chem. A 2019, 7, 12654–12660.
- Tang, Q.; Bairi, P.; Shrestha, R.G.; Hill, J.P.; Ariga, K.; Zeng, H.; Ji, Q.; Shrestha, L.K. Quasi 2D Mesoporous Carbon Microbelts Derived from Fullerene Crystals as an Electrode Material for Electrochemical Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 44458–44465.
