This is an efficient near-infrared (NIR) imaging method used to monitor VHH and VHH conjugated nanoparticle kinetics in mice using a hybrid approach: kinetics in blood were assessed by direct sampling, and kinetics in kidney, liver, and brain were assessed by serial in vivo NIR imaging. These studies were performed under “basal” circumstances in which the VHH constructs and VHH-conjugated nanoparticles do not substantially interact with targets nor cross the blood brain barrier.
- VHH
- nanoparticles
- near-infrared imaging
- pharmacokinetics
- modeling
 1. Introduction
1. Introduction
1.1 Neurological disorders
Neurological disorders affect millions of people worldwide, but at present our ability to assess these disorders objectively and quantitatively is limited [1]. Improvements in the assessment of neurological disorders would allow for disease progression monitoring and provide direct assessment of candidate therapeutics [2][3][4][5]. The long-term goal of this project is to develop MRI molecular contrast agents that will cross the blood brain barrier (BBB) and label relevant extracellular and intracellular biomarkers in the brain parenchyma. In the process of discovering and optimizing necessary components of these contrast agents, we have synthesized nanoparticles that consist of an iron oxide nanoparticle (IONP) core conjugated with Polyethylene glycol (PEG) plus single domain antibodies from camelids (VHH) for specific targeting.
1.2 IONP
IONPs have been widely used for medical applications including cancer diagnosis and treatment [6][7], treatment of iron deficiency anemia [6], enhanced blood pool and tumor MRI imaging [8][9], MRI molecular imaging [10][11][12][13], and magnetic resonance angiography (MRA) [14]. Sillerud et al. synthesized a novel MRI contrast agent by conjugating superparamagnetic oxide nanoparticles (SPIONs) with anti-amyloid-beta precursor protein (AβPP) antibodies to specifically target amyloid-beta plaques [15]. Iron oxide nanoparticles have also been functionalized with single-chain antibodies (scFv) against activated platelets for T1 and T2-weighted MRI of thrombi [10]. IONPs are considered relatively safe and do not induce cytotoxicity below 100 μg/mL in vitro [16]. MRI Molecular contrast agents based on iron oxide nanoparticles have good biocompatibility, at least in part be-cause human blood and tissues are naturally rich in iron [17][18]. The FDA has approved an IONP, Ferumoxytol, for treatment of iron-deficiency anemia in patients with chronic kidney disease [19]. Ferumoxytol is also used off-label as a contrast agent for MR angiography in patients with impaired renal function as well [20] and no major safety concerns have been reported. In a widely cited publication, Kim et al. [9] demonstrated that homogenous size iron oxide nanoparticle cores for MR imaging could be synthesized at large scales. Their extremely small 3 nm iron oxide nanoparticles (ESIONs) were shown to have a high r1 relaxivity of 4.78 mM−1s−1 at 3T and low r2/r1 ratio of 6.12, which maximizes the T1 contrast effect. ESIONs were tested using in vivo MRI. After tail vein injection of ESION (2.5 mg Fe/kg), blood vessels were brightened on the T1-weighted MR images, con-firming that ESIONs can enhance T1 relaxation and be used as a T1 MRI contrast agent. The iron oxide core of this prototype nanoparticle contrast agent was coated with PEG [21]. PEG is a common coating material that is used to prevent nanoparticle fouling in blood by reducing protein binding and to prolong circulation times by reducing clearance by the reticuloendothelial system (RES) [22].
1.3 HCAb and VHH
Camelids, which include llamas, alpacas, and camels, produce functional antibodies devoid of light chains called heavy chain-only antibodies (HCAbs) [23][24]. The heavy chain of this kind of antibody is folded into three domains: the N-terminal domain that is variable in sequence, followed by a hinge region and two constant domains. HCAbs recognize their cognate antigen by one single domain, the VHH. VHHs have a very small size compared to other antibodies or functional antibody fragments. The molecular weight of a VHH is approximately 15 kDa, which is around 1/10 of a conventional IgG’s molecular weight, and about 50% of that of a single chain variable fragment (ScFv) [25][26]. VHHs have been used for in vivo imaging and therapeutics [25][26]. For example, Li et al. labeled anti-Aβ42 and anti-Tau VHHs with Alexa488 fluorescent dye and visualized extracellular Aβ and intracellular neurofibrillary tangles using 2-photon-microscopy [27]. Vandesquille et al. conjugated a VHH (R3VQ)-targeting Aß with gadolinium to allow MRI detection of Aβ in post-mortem mouse brain [28]. Rincon et al. used VHHs to lower Aβ levels with AAV-based delivery of anti-BACE1 VHH into the CNS of a cerebral amyloidosis mouse model [29]. VHHs against SARS-CoV-2, which could bind spike protein receptor binding domain, were recently developed as potential therapeutics for coronavirus outbreaks [30][31][32][33]. A humanized divalent VHH targeting von Willebrand factor (Caplacizumab) was recently approved by the FDA for treatment of acquired thrombotic thrombocytopenic purpura [34][35]. Importantly, VHHs show low immunogenicity risk profile before humanization [36]. For human therapeutic purposes, VHHs have been humanized to further lower the risk of immunogenicity [37]. For example, the safety of Caplacizumab has generally been good [38].
1.4 Pharmacokinetics
Pharmacokinetics (PK) is the study of drug absorption, distribution, metabolism, and excretion [39]. Pharmacokinetic and biodistribution characteristics are important parameters to consider when designing and testing novel nanoparticles to achieve an appropriate level of nanoparticles in the target tissue site. Nanoparticles with either extremely short or extremely long circulation time are generally considered non-optimal; nanoparticles with extremely short circulation time may not have enough time to penetrate target tissue sites, while nanoparticles with extremely long circulation time could cause off-target tis-sue toxicity and reduce signal-to-noise ratio due to background signal [40][41][42][43].
1.5 Near infrared (NIR)
Near infrared (NIR) imaging has been proposed as an alternative method to study pharmacokinetics and tissue distribution to facilitate nanoparticle development [40]. Compared with the aforementioned methods, NIR is a less-expensive, faster, and safer method, which can be used to investigate nanoparticles’ in vivo behavior in appropriate small animal models [40]. PK models use a system of mathematical equations to describe drug pharmacokinetics.
2. Medical Use
2.1 VHH and VHH-Conjugated Nanoparticle Characterization
VHH singlet and VHH triplet products had characteristics consistent with expectation. Twenty-two 1 mL elution fractions were collected from a Superdex 75 size exclusion chromatography column. Peak fractions of purified VHH singlet and VHH triplet were collected for FNIR conjugation. VHH singlet peaked at fraction 11 and VHH triplet peaked at fraction 9 (Figure 1a). Based on the protein size standards, VHH singlet and VHH triplet eluted from the columns as expected based on their calculated sizes of 12.7 kDa and 36.1 kDa, respectively.
Based on the dynamic light scattering (DLS) data, the IONPPEG2000 particles had a hydrodynamic radius of 6.4–6.7 nm before and 7.0–7.6 nm after conjugation with VHH triplet (Figure 1c). The 9th and 10th fractions from a Superose 6 Increase SEC column (optimized for larger particles) were used for IV injection and PK studies (Figure 1b). The IONPPEG2000/750 VHH triplet constructs had hydrodynamic sizes of 8.3–9.1 nm (batch 1) and 8.0–9.1 nm (batch 2) before, and 11.9–13.9 nm (batch 1) and 12.2–14.5 nm (batch 2) after conjugation with VHH triplet. The fifth, sixth, and seventh fractions from batch 1 and the sixth and seventh fractions from batch 2 from the Superose 6 Increase column were used for IV injection and PK studies (Figure 1b).
2.2 In Vivo Pharmacokinetic Study Using NIR
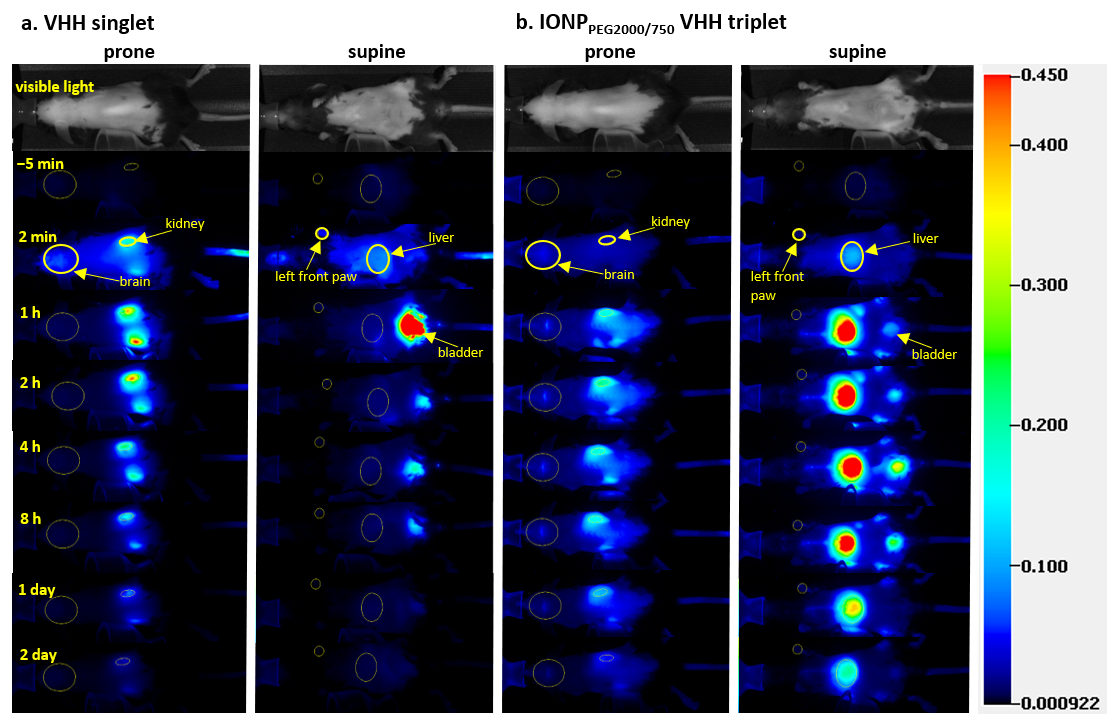
We optimized methods for NIR-based pharmacokinetic studies and then used these methods to acquire consistent in vivo pharmacokinetic data in mice. NIR images of in vivo mice injected with four different FNIR dye conjugates (two VHHs and two IONPs) showed that the conjugates had different biodistributions in brain, kidney, and liver and were cleared from mice at different rates (Figure 2). Regions of interest (ROIs) of major organs including brain, kidney, and liver were drawn on the images acquired before, 2 min, 1 h, 2 h, 4 h, 8 h, 1 day, and 2 days after IV injection at both prone and supine positions of mice (Figure 2a for VHH singlet, Figure 2b for IONPPEG2000/750 VHH triplet). For VHH singlet, the pharmacokinetic plots of kidney and liver showed that kidneys and liver had similar uptake, but kidneys had much slower fluorescence intensity decrease rate than liver, indicating that kidneys were the major organ for VHH singlet clearance (Figure 3a). For VHH triplet, the pharmacokinetic plots of kidney and liver showed that kidneys and liver had similar uptake and clearance rates, indicating that kidneys and liver both contribute to the clearance (Figure 3b). For IONPPEG2000 VHH triplet and IONPPEG2000/750 VHH triplet, the pharmacokinetic plots of kidney and liver showed that liver had much larger uptake of nanoparticles than kidneys, indicating that liver was the main organ for the IONP-VHH conjugate clearance (Figure 3c,d). There were only modest differences in kinetics between the two different PEG coatings that were used to make the IONPs water soluble and serve as linkers for VHH conjugations. Particles coated with PEG2000 vs. particles coated with a 1:1 ratio of PEG2000 to PEG750 had similar kinetics with predominant liver uptake and biphasic clearance from the brain compartment.
2.3 Blood Clearance Measured by NIR
We adopted a hybrid approach and measured PK in blood by direct sampling of blood from separate groups of mice sacrificed at multiple time points. Blood was sampled at 1 min, 5 min, 10 min, 15 min, 30 min, and 1 h after IV injection and quantified using ROIs drawn on the NIR images of the ex vivo blood. The blood clearance curves were fitted using a single exponential equation. The NIR fluorescence images of ex vivo blood indicated fast blood clearance rate of VHHs and slower rate of VHH-IONP. Most of the materials were cleared out of the blood within the first 1 h post injection. The fitted half-lives were 7.09 min, 2.86 min, and 1.94 min for IONPPEG2000 VHH triplet, VHH triplet, and VHH singlet respectively.
3. Limitation
The signal from NIR images does not reflect the absolute concentrations in the compartments of interest. The presence of skin and soft tissues reduces image quality by optical attenuation and scattering [44][45]. In addition, NIR methods are less amenable to assessing smaller compartments such as spleen or bone marrow. Clearly, NIR approaches are best suited for relatively short-term studies in small animals such as mice with little intrinsic skin pigmentation; in larger animals there is too much attenuation between the tissues of interest and the detectors. Longer term PK studies would be difficult because of the challenges of maintaining hair removal for more than a few days without compromising health. Another substantial limitation is that we only characterized PK in relation to construct size. There are many other properties of IONPs including hydrophobicity, surface charge, and coating or conformation of nanoparticles/VHHs that could affect their PK and biodistribution [6][8][22][46].
This entry is adapted from the peer-reviewed paper 10.3390/ijms22168695
References
- Valery L Feigin; Emma Nichols; Tahiya Alam; Marlena S Bannick; Ettore Beghi; Natacha Blake; William J Culpepper; E Ray Dorsey; Alexis Elbaz; Richard G Ellenbogen; et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology 2019, 18, 459-480, 10.1016/s1474-4422(18)30499-x.
- Michael G. Erkkinen; Mee-Ohk Kim; Michael D. Geschwind; Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harbor Perspectives in Biology 2017, 10, a033118, 10.1101/cshperspect.a033118.
- Nithya Srinivas; Kaitlyn Maffuid; Angela D. M. Kashuba; Clinical Pharmacokinetics and Pharmacodynamics of Drugs in the Central Nervous System. Clinical Pharmacokinetics 2018, 57, 1059-1074, 10.1007/s40262-018-0632-y.
- Vinay Sridhar; Ram Gaud; Amrita Bajaj; Sarika Wairkar; Pharmacokinetics and pharmacodynamics of intranasally administered selegiline nanoparticles with improved brain delivery in Parkinson's disease. Nanomedicine: Nanotechnology, Biology and Medicine 2018, 14, 2609-2618, 10.1016/j.nano.2018.08.004.
- Silvia Basaia; Federica Agosta; Luca Wagner; Elisa Canu; Giuseppe Magnani; Roberto Santangelo; Massimo Filippi; Automated classification of Alzheimer's disease and mild cognitive impairment using a single MRI and deep neural networks. NeuroImage: Clinical 2018, 21, 101645, 10.1016/j.nicl.2018.101645.
- Edouard Alphandéry; Biodistribution and targeting properties of iron oxide nanoparticles for treatments of cancer and iron anemia disease. Nanotoxicology 2019, 13, 573-596, 10.1080/17435390.2019.1572809.
- I.T. Rubio; S. Diaz-Botero; Antonio Esgueva; R. Rodriguez; Tomas Cortadellas; O. Cordoba; Martin Espinosa-Bravo; The superparamagnetic iron oxide is equivalent to the Tc99 radiotracer method for identifying the sentinel lymph node in breast cancer. European Journal of Surgical Oncology (EJSO) 2014, 41, 46-51, 10.1016/j.ejso.2014.11.006.
- Dan Ma; Jingwen Chen; Yu Luo; Han Wang; Xiangyang Shi; Zwitterion-coated ultrasmall iron oxide nanoparticles for enhanced T1-weighted magnetic resonance imaging applications. Journal of Materials Chemistry B 2017, 5, 7267-7273, 10.1039/c7tb01588g.
- Byung Hyo Kim; Nohyun Lee; Hyoungsu Kim; Kwangjin An; Yong Il Park; Yoonseok Choi; Kwangsoo Shin; Youjin Lee; Soon Gu Kwon; Hyon Bin Na; et al. Large-Scale Synthesis of Uniform and Extremely Small-Sized Iron Oxide Nanoparticles for High-Resolution T1 Magnetic Resonance Imaging Contrast Agents. Journal of the American Chemical Society 2011, 133, 12624-12631, 10.1021/ja203340u.
- Hang T. Ta; Zhen Li; Christoph E. Hagemeyer; Gary Cowin; Shaohua Zhang; Jathushan Palasubramaniam; Karen Alt; Xiaowei Wang; Karlheinz Peter; Andrew K. Whittaker; et al. Molecular imaging of activated platelets via antibody-targeted ultra-small iron oxide nanoparticles displaying unique dual MRI contrast. Biomaterials 2017, 134, 31-42, 10.1016/j.biomaterials.2017.04.037.
- Matthias Nahrendorf; Farouc A. Jaffer; Kimberly A. Kelly; David E. Sosnovik; Elena Aikawa; Peter Libby; Ralph Weissleder; Noninvasive Vascular Cell Adhesion Molecule-1 Imaging Identifies Inflammatory Activation of Cells in Atherosclerosis. Circulation 2006, 114, 1504-1511, 10.1161/circulationaha.106.646380.
- Michael Barrow; Arthur Taylor; Patricia Murray; Matthew J. Rosseinsky; Dave J. Adams; Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI. Chemical Society Reviews 2015, 44, 6733-6748, 10.1039/c5cs00331h.
- Carlos Tarin; Monica Carril; Jose Luis Martin-Ventura; Irati Markuerkiaga; Daniel Padro; Patricia Llamas-Granda; Juan Antonio Moreno; Isabel García; Nuria Genicio; Sandra Plaza-Garcia; et al. Targeted gold-coated iron oxide nanoparticles for CD163 detection in atherosclerosis by MRI. Scientific Reports 2015, 5, 17135-17135, 10.1038/srep17135.
- He Wei; Oliver T. Bruns; Michael G. Kaul; Eric C. Hansen; Mariya Barch; Agata Wiśniowska; Ou Chen; Yue Chen; Nan Li; Satoshi Okada; et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proceedings of the National Academy of Sciences 2017, 114, 2325-2330, 10.1073/pnas.1620145114.
- Laurel O. Sillerud; Nathan O. Solberg; Ryan Chamberlain; Robert A. Orlando; John E. Heidrich; David C. Brown; Christina I. Brady; Thomas A. Vander Jagt; Michael Garwood; David L. Vander Jagt; et al. SPION-Enhanced Magnetic Resonance Imaging of Alzheimer's Disease Plaques in AβPP/PS-1 Transgenic Mouse Brain. Journal of Alzheimer's Disease 2013, 34, 349-365, 10.3233/JAD-121171.
- Neenu Singh; Gareth Jenkins; Romisa Asadi; Shareen Doak; Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Reviews 2009, 1, 5358, 10.3402/nano.v1i0.5358.
- Domingo J. Piñero; James R. Connor; Iron in the Brain: An Important Contributor in Normal and Diseased States. The Neuroscientist 2000, 6, 435-453, 10.1177/107385840000600607.
- Stefan Ropele; Christian Langkammer; Iron quantification with susceptibility. NMR in Biomedicine 2016, 30, e3534, 10.1002/nbm.3534.
- Food and Drug Administration. Feraheme (Ferumoxytol) Information. Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/feraheme-ferumoxytol-information (accessed on June 30 2009).
- Gerda B. Toth; Csanad G. Varallyay; Andrea Horvath; Mustafa R. Bashir; Peter L. Choyke; Heike E. Daldrup-Link; Edit Dosa; John Paul Finn; Seymur Gahramanov; Mukesh Harisinghani; et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney International 2017, 92, 47-66, 10.1016/j.kint.2016.12.037.
- Hyon Bin Na; In Su Lee; Heonjin Seo; Yong Il Park; Jung Hee Lee; Sang-Wook Kim; Taeghwan Hyeon; Versatile PEG-derivatized phosphine oxide ligands for water-dispersible metal oxide nanocrystals. Chemical Communications 2007, N/A, 5167-5169, 10.1039/b712721a.
- Jinbin Liu; Mengxiao Yu; Xuhui Ning; Chen Zhou; Shengyang Yang; Dr. Jie Zheng; PEGylation and Zwitterionization: Pros and Cons in the Renal Clearance and Tumor Targeting of Near-IR-Emitting Gold Nanoparticles. Angewandte Chemie International Edition 2013, 52, 12572-12576, 10.1002/anie.201304465.
- C. Hamers-Casterman; T. Atarhouch; Serge Muyldermans; G. Robinson; C. Hammers; E. Bajyana Songa; N. Bendahman; Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446-448, 10.1038/363446a0.
- Serge Muyldermans; Applications of Nanobodies. Annual Review of Animal Biosciences 2021, 9, 401-421, 10.1146/annurev-animal-021419-083831.
- Gholamreza Hassanzadeh-Ghassabeh; Nick Devoogdt; Pieter De Pauw; Cécile Vincke; Serge Muyldermans; Nanobodies and their potential applications. Nanomedicine 2013, 8, 1013-1026, 10.2217/nnm.13.86.
- M. M. Harmsen; H. J. De Haard; Properties, production, and applications of camelid single-domain antibody fragments. Applied Microbiology and Biotechnology 2007, 77, 13-22, 10.1007/s00253-007-1142-2.
- Tengfei Li; Matthias Vandesquille; Fani Koukouli; Clémence Dudeffant; Ihsen Youssef; Pascal Lenormand; Christelle Ganneau; Uwe Maskos; Christian Czech; Fiona Grueninger; et al. Camelid single-domain antibodies: A versatile tool for in vivo imaging of extracellular and intracellular brain targets. Journal of Controlled Release 2016, 243, 1-10, 10.1016/j.jconrel.2016.09.019.
- Matthias Vandesquille; Tengfei Li; Chrystelle Po; Christelle Ganneau; Pascal Lenormand; Clémence Dudeffant; Christian Czech; Fiona Grueninger; Charles Duyckaerts; Benoît Delatour; et al. Chemically-defined camelid antibody bioconjugate for the magnetic resonance imaging of Alzheimer's disease. mAbs 2017, 9, 1016-1027, 10.1080/19420862.2017.1342914.
- Melvin Y. Rincon; Lujia Zhou; Catherine Marneffe; Iryna Voytyuk; Yessica Wouters; Maarten Dewilde; Sandra I. Duqué; Cécile Vincke; Yona Levites; Todd E. Golde; et al. AAV mediated delivery of a novel anti-BACE1 VHH reduces Abeta in an Alzheimer’s disease mouse model. null 2019, N/A, N/A, 10.1101/698506.
- Thomas J. Esparza; Negin P. Martin; George P. Anderson; Ellen R. Goldman; David L. Brody; High affinity nanobodies block SARS-CoV-2 spike receptor binding domain interaction with human angiotensin converting enzyme. Scientific Reports 2020, 10, 1-13, 10.1038/s41598-020-79036-0.
- Jiandong Huo; Audrey Le Bas; Reinis R. Ruza; Helen M. E. Duyvesteyn; Halina Mikolajek; Tomas Malinauskas; Tiong Kit Tan; Pramila Rijal; Maud Dumoux; Philip N. Ward; et al. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nature Structural & Molecular Biology 2020, 27, 846-854, 10.1038/s41594-020-0469-6.
- Jianbo Dong; Betty Huang; Bo Wang; Allison Titong; Sachith Gallolu Kankanamalage; Zhejun Jia; Meredith Wright; Pannaga Parthasarathy; Yue Liu; Development of humanized tri-specific nanobodies with potent neutralization for SARS-CoV-2. Scientific Reports 2020, 10, 1-12, 10.1038/s41598-020-74761-y.
- Michael Schoof; Bryan Faust; Reuben A. Saunders; Smriti Sangwan; Veronica Rezelj; Nick Hoppe; Morgane Boone; Christian B. Billesbølle; Cristina Puchades; Caleigh M. Azumaya; et al. An ultrapotent synthetic nanobody neutralizes SARS-CoV-2 by stabilizing inactive Spike. Science 2020, 370, 1473-1479, 10.1126/science.abe3255.
- Marie Scully; Spero R. Cataland; Flora Peyvandi; Paul Coppo; Paul Knoebl; Johanna A. Kremer Hovinga; Ara Metjian; Javier De La Rubia; Katerina Pavenski; Filip Callewaert; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. New England Journal of Medicine 2019, 380, 335-346, 10.1056/nejmoa1806311.
- Food and Drug Administration. FDA Approved Caplacizumab-yhdp. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approved-caplacizumab-yhdp (accessed on February 6 2019).
- Chloé Ackaert; Natalia Smiejkowska; Catarina Xavier; Yann G. J. Sterckx; Sofie Denies; Benoit Stijlemans; Yvon Elkrim; Nick Devoogdt; Vicky Caveliers; Tony Lahoutte; et al. Immunogenicity Risk Profile of Nanobodies. Frontiers in Immunology 2021, 12, N/A, 10.3389/fimmu.2021.632687.
- Cécile Vincke; Remy Loris; Dirk Saerens; Sergio Martinez-Rodriguez; Serge Muyldermans; Katja Conrath; General Strategy to Humanize a Camelid Single-domain Antibody and Identification of a Universal Humanized Nanobody Scaffold. Journal of Biological Chemistry 2008, 284, 3273-3284, 10.1074/jbc.m806889200.
- Md Paul Knöbl; Md Mrcp Marie Scully; Spero R Cataland; Md Flora Peyvandi; Md Paul Coppo; Md Johanna A. Kremer Hovinga; Ara Metjian; Javier De La Rubia; Katerina Pavenski; Jessica Minkue; et al. Integrated Safety Results from the Phase II and Phase III Studies with Caplacizumab in Patients with Acquired Thrombotic Thrombocytopenic Purpura. Blood 2018, 132, 3739-3739, 10.1182/blood-2018-99-112174.
- Ratain, M.J.; Plunkett, W.K., Jr.. Principles of Pharmacokinetics.; BC Decker: Hamilton, ON, Canada, 2003; pp. N/A.
- Michelle Jeung-Eun Lee; Omid Veiseh; Narayan Bhattarai; Conroy Sun; Stacey J. Hansen; Sally Ditzler; Sue Knoblaugh; Nghoon Lee; Richard Ellenbogen; Miqin Zhang; et al. Rapid Pharmacokinetic and Biodistribution Studies Using Cholorotoxin-Conjugated Iron Oxide Nanoparticles: A Novel Non-Radioactive Method. PLoS ONE 2010, 5, e9536, 10.1371/journal.pone.0009536.
- Nazanin Hoshyar; Samantha Gray; HongBin Han; Gang Bao; The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673-692, 10.2217/nnm.16.5.
- Homan Kang; Shrutika Mintri; Archita Venugopal Menon; Hea Yeon Lee; Hak Soo Choi; Jonghan Kim; Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale 2015, 7, 18848-18862, 10.1039/c5nr05264e.
- Sheng Tong; Sijian Hou; Zhilan Zheng; Jun Zhou; Gang Bao; Coating Optimization of Superparamagnetic Iron Oxide Nanoparticles for High T2 Relaxivity. Nano Letters 2010, 10, 4607-4613, 10.1021/nl102623x.
- Atif Zaheer; Robert Lenkinski; Ashfaq Mahmood; Alun G. Jones; Lewis Cantley; John V. Frangioni; In vivo near-infrared fluorescence imaging of osteoblastic activity. Nature Biotechnology 2001, 19, 1148-1154, 10.1038/nbt1201-1148.
- Caerwyn Ash; Michael Dubec; Kelvin Donne; Tim Bashford; Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers in Medical Science 2017, 32, 1909-1918, 10.1007/s10103-017-2317-4.
- Weiming Xue; Yanyan Liu; Na Zhang; Youdong Yao; Pei Ma; Huiyun Wen; Saipeng Huang; Yan E Luo; Haiming Fan; Effects of core size and PEG coating layer of iron oxide nanoparticles on the distribution and metabolism in mice. International Journal of Nanomedicine 2018, ume 13, 5719-5731, 10.2147/ijn.s165451.
