Anaerobic digestion is associated with various crucial variables, such as biogas yield, chemical oxygen demand, and volatile fatty acid concentration. Real-time monitoring of these variables can not only reflect the process of anaerobic digestion directly but also accelerate the efficiency of resource conversion and improve the stability of the reaction process. Therefore, it is essential to conduct soft sensor modeling for unmeasurable variables and use auxiliary variables to realize real-time monitoring, optimization, and control of the an-aerobic digestion process.
- anaerobic digestion
- soft sensor
- deep learning
1. Introduction
2. Anaerobic Digestion Process
2.1. Basic Principles of Anaerobic Digestion
2.2. Process Parameters of Anaerobic Digestion
-
PH: The optimal pH range of different microorganisms is different. Methanogens are extremely sensitive to pH, and the optimal pH range is 6.5–7.2 [30]. The fermenting microorganisms produce acetic acid and butyric acid when the pH is low. Acetic acid and propionic acid are formed when the pH is higher than 8.0 [31]. Therefore, reasonable monitoring of pH can ensure the maximum biological activity of microorganisms.
-
Alkalinity: Methanogens usually produce alkalinity in the form of carbon dioxide, ammonia, and bicarbonate, contributing to neutralizing VFA produced during anaerobic digestion [32]. Thus, real-time monitoring of alkalinity can improve the stability of the anaerobic digestion process when the concentration of carbon dioxide is stable.
-
Temperature: Temperature has a crucial influence on the physical and chemical properties of anaerobic digestion and fermentation substrates. It affects the growth rate and metabolism of microorganisms, which in turn influences the population dynamics of the anaerobic digestion process [33]. When the temperature changes more than 1 °C/day, the biochemical activity of methanogens will be severely affected, causing the process to fail.
-
VFA concentration: VFA concentration is an intermediate product of the anaerobic digestion process. Excessive accumulation of VFA can reduce the pH of the system and inhibit the activity of methanogens. The VFA concentration can reflect the current operating conditions of the system while being extremely sensitive to the incoming feed imbalance [34]. Hence, it is urgent to establish a soft sensor to predict the VFA concentration by monitoring the measurable and easy-to-obtain process variables in real-time.
-
COD and biogas yield: COD is an imperative indicator to measure the organic content of the effluent from the anaerobic digestion process [35]. Biogas yield is a vital indicator to measure the efficiency of anaerobic digestion [36]. Real-time monitoring of COD and biogas yield can demonstrate the operating efficiency and stability of the anaerobic digestion process and contribute to achieving the real-time calibration and optimization of production conditions and control methods.
2.3. Anaerobic Digestion Process
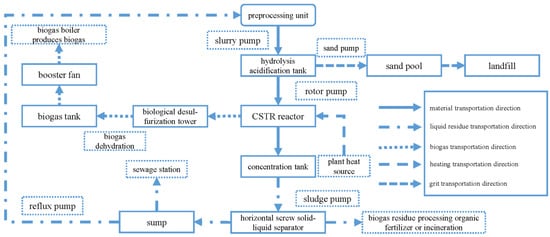
3. The Latest Development of Anaerobic Digestion Soft Sensor
|
Soft Sensors |
Advantages of Soft Sensor |
Defects of Soft Sensor |
|---|---|---|
|
Soft sensor based on process mechanism |
High precision, strong interpretability, clear industrial background |
It is difficult to build an accurate mechanism model |
|
Soft sensor based on state estimation |
Solve the problem of dynamic characteristic differences and system lag between variables |
Simplifying the system will increase forecast errors |
|
Soft sensor based on MLR |
Only consider the mapping relationship of data; do not require a clear internal mechanism |
The accuracy is not high, and it is easily affected by external interference |
|
Soft sensor based on PLSR |
Solve the problem of collinearity between auxiliary variables |
Inability to handle strong nonlinear problems |
|
Soft sensor based on BP neural network |
Able to achieve an arbitrary precision approximation of nonlinear functions |
Easy to fall into local optimal or over-fitting state |
|
Soft sensor based on RBF neural network |
Realize the global best approximation and solve the local optimal problem |
Affected by network topology and hyperparameters |
|
Soft sensor based on SVR |
Solve the problem of high dimensions and small samples |
Unable to handle large-scale data |
|
Soft sensor based on LS-SVR |
Further reduce the complexity of the model and increase the calculation speed |
Very sensitive to outliers and poor robustness |
-
The traditional soft sensor cannot extract the deep features of auxiliary variables. The performance of traditional soft sensors depends on the auxiliary variables provided, and the selection of auxiliary variables requires rich prior knowledge [46].
-
The traditional soft sensor does not consider the large number of unlabeled samples in the anaerobic digestion process. There are many unlabeled samples in the anaerobic digestion process. The semi-supervised learning mechanism, which is used to mine unlabeled sample information, can effectively improve the prediction performance of soft sensors [47].
-
The traditional soft sensor does not consider the dynamic and time lag characteristics of anaerobic digestion. The traditional soft sensor cannot adapt to changes in work and production conditions, and the prediction accuracy of the soft sensor gradually deteriorates over time [48]. Meanwhile, the slow hydrolysis process of anaerobic digestion would lead to a certain time lag between the real-time monitoring variables of the acid-producing tank and the real-time monitoring variables of the methane-producing tank.
-
The traditional soft sensor only considers the mapping relationship between auxiliary variables and target variables while ignoring the mutual influence between auxiliary variables [49]. In the actual industry, the combined auxiliary variables are generally highly correlated with the target variable while the single auxiliary variable often has a weak correlation with the target variable.
This entry is adapted from the peer-reviewed paper 10.3390/pr9081434
References
- Yang, H.; Mo, W.-l.; Xiong, Z.-X.; Huang, M.-Z.; Liu, H.-B. Soft sensor modeling of papermaking effluent treatment processes using RPLS. China Pulp Pap. 2018, 35, 31–35.
- Yordanova, S.; Noikova, N.; Petrova, R.; Tzvetkov, P. Neuro-fuzzy modelling on experimental data in anaerobic digestion of organic waste in waters. In Proceedings of the 2005 IEEE Intelligent Data Acquisition and Advanced Computing Systems: Technology and Applications, Sofia, Bulgaria, 5–7 September 2005; pp. 84–88.
- Liu, Z.-J.; Wan, J.-Q.; Ma, Y.-W.; Wang, Y. Online prediction of effluent COD in the anaerobic wastewater treatment system based on PCA-LSSVM algorithm. Env. Sci. Pollut. Res. Int. 2019, 26, 12828–12841.
- Bryant, M.P.; Wolin, E.A.; Wolin, M.J.; Wolfe, R.S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch. Mikrobiol. 1967, 59, 20–31.
- Bryant, M.P. Microbial methane production—theoretical aspects. J. Anim. Sci. 1979, 48, 193–201.
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag. 2014, 34, 2080–2089.
- Sbarciog, M.; Loccufier, M.; Noldus, E. Determination of appropriate operating strategies for anaerobic digestion systems. Biochem. Eng. J. 2010, 51, 180–188.
- Shen, S.; Premier, G.C.; Guwy, A.; Dinsdale, R. Bifurcation and stability analysis of an anaerobic digestion model. Nonlinear Dyn. 2007, 48, 391–408.
- Lara-Cisneros, G.; Aguilar-López, R.; Femat, R. On the dynamic optimization of methane production in anaerobic digestion via extremum-seeking control approach. Comput. Chem. Eng. 2015, 75, 49–59.
- Corona, F.; Mulas, M.; Haimi, H.; Sundell, L.; Heinonen, M.; Vahala, R. Monitoring nitrate concentrations in the denitrifying post-filtration unit of a municipal wastewater treatment plant. J. Process Control 2013, 23, 158–170.
- Jimenez, J.; Latrille, E.; Harmand, J.; Robles, A.; Ferrer, J.; Gaida, D.; Wolf, C.; Mairet, F.; Bernard, O.; Alcaraz-Gonzalez, V.; et al. Instrumentation and control of anaerobic digestion processes: A review and some research challenges. Rev. Environ. Sci. Biotechnol. 2015, 14, 615–648.
- Kawai, M.; Nagao, N.; Kawasaki, N.; Imai, A.; Toda, T. Improvement of COD removal by controlling the substrate degradability during the anaerobic digestion of recalcitrant wastewater. J. Environ. Manag. 2016, 181, 838–846.
- Gaida, D.; Wolf, C.; Meyer, C.; Stuhlsatz, A.; Lippel, J.; Bäck, T.; Bongards, M.; McLoone, S. State estimation for anaerobic digesters using the ADM1. Water Sci. Technol. 2012, 66, 1088–1095.
- Haimi, H.; Mulas, M.; Corona, F.; Vahala, R. Data-derived soft-sensors for biological wastewater treatment plants: An overview. Environ. Model. Softw. 2013, 47, 88–107.
- Gaida, D.; Wolf, C.; Bongards, M. Feed control of anaerobic digestion processes for renewable energy production: A review. Renew. Sustain. Energy Rev. 2017, 68, 869–875.
- Langergraber, G.; Fleischmann, N.; Hofstaedter, F.; Weingartner, A. Monitoring of a paper mill wastewater treatment plant using UV/VIS spectroscopy. Water Sci. Technol. 2004, 49, 9–14.
- Han, D.; Zou, Z. Soft sensor and inferential control technology. J. Nanjing Univ. Sci. Technol. 2005, 212–216.
- Wade, M.J. Not just numbers: Mathematical modelling and its contribution to anaerobic digestion processes. Processes 2020, 8, 888.
- Brosilow, C.; Tong, M. Inferential control of processes: Part II. The structure and dynamics of inferential control systems. AIChE 1978, 24, 492–500.
- He, B.; Zhu, X. Soft-sensing technique based on extension method. In Proceedings of the SPIE 5253, Fifth International Symposium on Instrumentation and Control Technology, Beijing, China, 24–27 October 2003; pp. 38–42.
- Wang, Z.-x.; Liu, Z.-w.; Xue, F.-x. Soft sensing technique for sewage treatment process. J. Beijing Technol. Bus. Univ. 2005, 23, 31–34.
- Yu, J.; Zhou, C. Soft-sensing techniques in process control. Control Theory Appl. 1996, 137–144.
- Zhu, X. Soft-sensing technique and its applications. J. South China Univ. Technol. 2002, 30, 61–67.
- James, S.C.; Legge, R.L.; Budman, H. On-line estimation in bioreactors: A review. Rev. Chem. Eng. 2000, 16, 311–340.
- Kadlec, P.; Gabrys, B.; Strandt, S. Data-driven soft sensors in the process industry. Comput. Chem. Eng. 2009, 33, 795–814.
- Zeikus, J. Microbial populations in digesters. In Proceedings of the First International Symposium on Anaerobic Digestion, London, UK, 17–21 September 1979.
- Keymer, P.; Ruffell, I.; Pratt, S.; Lant, P. High pressure thermal hydrolysis as pre-treatment to increase the methane yield during anaerobic digestion of microalgae. Bioresour. Technol. 2013, 131, 128–133.
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781.
- Illi, L.; Lecker, B.; Lemmer, A.; Müller, J.; Oechsner, H. Biological methanation of injected hydrogen in a two-stage anaerobic digestion process. Bioresour. Technol. 2021, 333, 125126.
- Kazemi, P.; Bengoa, C.; Steyer, J.-P.; Giralt, J. Data-driven techniques for fault detection in anaerobic digestion process. Process Saf. Environ. Prot. 2021, 146, 905–915.
- Boe, K. Online Monitoring and Control of the Biogas Process; Institute of Environment & Resources, Technical University of Denmark: Copenhagen, Denmark, 2006.
- Hwang, M.H.; Jang, N.J.; Hyun, S.H.; Kim, I.S. Anaerobic bio-hydrogen production from ethanol fermentation: The role of pH. J. Biotechnol. 2004, 111, 297–309.
- Stichting Toegepast Onderzoek Reiniging Afvalwater. Optimalisatie van de Gistingsgasproduktie; Stora: Amsterdam, The Netherlands, 1985.
- Turovskiy, I.S.; Mathai, P. Wastewater Sludge Processing; John Wiley & Sons: Hoboken, NJ, USA, 2006.
- Steyer, J.P.; Bouvier, J.C.; Conte, T.; Gras, P.; Harmand, J.; Delgenes, J.P. On-line measurements of COD, TOC, VFA, total and partial alkalinity in anaerobic digestion processes using infra-red spectrometry. Water Sci. Technol. 2002, 45, 133–138.
- Chae, K.J.; Jang, A.; Yim, S.K.; Kim, I.S. The effects of digestion temperature and temperature shock on the biogas yields from the mesophilic anaerobic digestion of swine manure. Bioresour. Technol. 2008, 99, 1–6.
- Massi, E. Anaerobic digestion. In Fuel Cells in the Waste-to-Energy Chain: Distributed Generation through Non-Conventional Fuels and Fuel Cells; McPhail, S.J., Cigolotti, V., Moreno, A., Eds.; Springer: London, UK, 2012; pp. 47–63.
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076.
- Liu, X.; Han, Z.; Yang, J.; Ye, T.; Yang, F.; Wu, N.; Bao, Z. Review of enhanced processes for anaerobic digestion treatment of sewage sludge. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 012039.
- Ye, N.-F.; He, P.-J.; Lü, F.; Shao, L.-M. Effect of pH on microbial diversity and product distribution during anaerobic fermentation of vegetable waste. Chin. J. Appl. Environ. Biol. 2007, 13, 238–242.
- Adekunle, K.F.; Okolie, J.A. A review of biochemical process of anaerobic digestion. Adv. Biosci. Biotechnol. 2015, 6, 205–212.
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744.
- Lettinga, G. Anaerobic digestion and wastewater treatment systems. Antonie Van Leeuwenhoek 1995, 67, 3–28.
- Mumme, J.; Linke, B.; Tölle, R. Novel upflow anaerobic solid-state (UASS) reactor. Bioresour. Technol. 2010, 101, 592–599.
- Angelidaki, I.; Chen, X.; Cui, J.; Kaparaju, P.; Ellegaard, L. Thermophilic anaerobic digestion of source-sorted organic fraction of household municipal solid waste: Start-up procedure for continuously stirred tank reactor. Water Res. 2006, 40, 2621–2628.
- Du, X.; Cai, Y.; Wang, S.; Zhang, L. Overview of deep learning. In Proceedings of the 31st Youth Academic Annual Conference of Chinese Association of Automation (YAC), Wuhan, China, 11–13 November 2016; pp. 159–164.
- Yao, L.; Ge, Z. Deep learning of semisupervised process data with hierarchical extreme learning machine and soft sensor application. ITIE 2018, 65, 1490–1498.
- Yuan, X.; Li, L.; Shardt, Y.A.W.; Wang, Y.; Yang, C. Deep learning with spatiotemporal attention-based LSTM for industrial soft sensor model development. ITIE 2021, 68, 4404–4414.
- Cao, Y.; Liu, C.; Huang, Z.; Sheng, Y.; Ju, Y. Skeleton-based action recognition with temporal action graph and temporal adaptive graph convolution structure. Multimed. Tools Appl. 2021.
