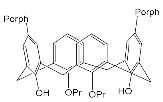
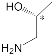
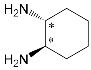
An important direction in the design of tetrapyrrole macrocyclic receptors for a certain substrate type is modification of the macrocycle periphery with bulky substituents or molecular fragments of different natures. Bulky highly-branched lateral substituents are capable of forming additional complexing cavities that can be used for identification and selective binding of substrates of a certain type.
- porphyrin
- receptor
- molecular recognition
- host–guest interactions
1. Introduction
Molecular recognition is a process in which host molecules (receptors) select and bind guest molecules (substrates) into structurally highly organized systems through multipoint intermolecular interactions [1,2,3,4,5,6,7]. Porphyrins are extremely useful for this purpose. Their unique properties are the result of the unusual geometric and electronic structure of the porphyrin macrocycle, containing a developed aromatic conjugated system. Selective chemical modification of porphyrins by fragments of other classes of compounds makes it possible to synthesize molecular systems that are different in their nature and positions of the reaction centers relative to each other [8,9,10,11,12,13,14]. This review discusses the progress made so far in the design and sensing and binding abilities of porphyrins modified with calix[4]arenes, calix[4]pyrroles, oxacalix-[2]-arenes-[2], and resorcinarenes and with bulky carbazolylphenyl substituents of different generations towards substrates of different natures. The molecular structures, sensing mechanism, and successful applications of these macrocyclic compounds with well-defined geometries as heterotopic receptors for molecular recognition and reversible binding of anions, cations, and small organic molecules are discussed in detail. Data on the ion-assisted binding ability of tetrapyrrole macroheterocyclic compounds decorated with additional complexing cavities and channels are of particular interest. It is shown that the binding ability of the porphyrin core depends on the conformation mobility of the substituents and the possibility of formation of intramolecular cavities and channels of various shapes within them.
2. Molecular Receptors Based on Porphyrin Conjugates with Macrocyclic Compounds of Different Natures
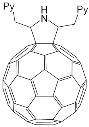
Interest in the chemistry of porphyrins was first sparked by the participation of these compounds in photosynthesis. However, it has now turned in another direction as porphyrins can be used as selective molecular receptors for certain types of substrates [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. The fact that it is possible to chemically modify the tetrapyrrole molecule has enabled researchers to synthesize porphyrin conjugates with other macrocyclic compounds(porphyrin conjugates with calix[n]arenes [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54], cyclodextrines [55,56,57,58,59,60,61,62,63,64,65,66], calix[n]pyrroles, fullerenes, ferrocenes, and resorcinarenes [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80]) possessing their own properties, allowing them to form complexes with ions and organic molecules of different natures. Table 1 and Table 2 present some data on “molecular tweezer” macrocyclic receptors and the substrates they bind, based on results of the analysis of the most interesting works in this field.
| “Molecular Tweezers” | Substrates | Ref. | “Molecular Tweezers” | Substrates | Ref. | ||
|---|---|---|---|---|---|---|---|
| MPorph. | Linked | MPorph. | Linked | ||||
| H2Porph.1 ZnPorph.2 |
 |
 |
[36] [37] |
H2Porph.1 | 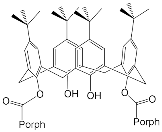 |
 |
[38] |
| H2Porph.1 | 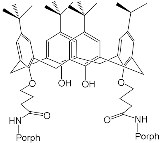 |
 |
[38] | H2Porph.1 H2Porph.3 H2Porph.4 |
 |
 |
[38] |
| ZnPorph.6 | 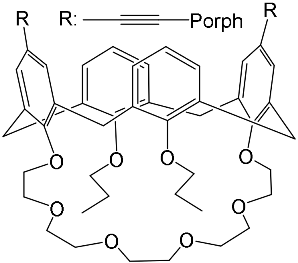 |
 |
[39] | ZnPorph.7 | 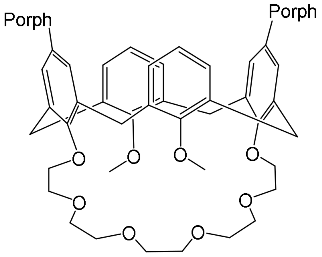 |
 |
[40] |
| ZnPorph.7 | 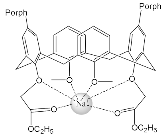 |
 |
[41] | H2Porph.8 | 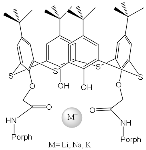 |
Regulation of the interporphyrin distance by the introduction of metal cations | [42] |
| ZnPorph.3 | 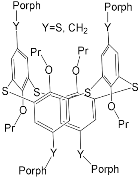 |
 |
[43] | ZnPorph.3 |  |
 |
[44] |
| H2Porph.9 |  |
Anions Cl−, Br−, I−, NO3− |
[45] | H2Porph.10 ZnPorph.10 |
 |
Fluorescent detection of Cu(II), Al(III), Cr(III), Zn(II), Cd(II) | [46] |
| ZnPorph.11 | 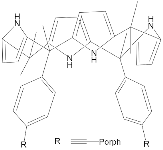 |
 |
[67] | ZnPorph.9 | 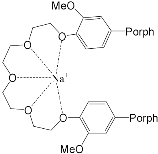 |
 |
[68] |
| ZnPorph.3 |  |
H2Porph.12 | [71] | ZnPorph.3 |  |
H2Porph.12 | [70] |
| ZnPorph.6 |  |
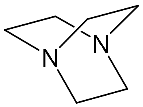
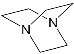
DABCO | [71] | ZnPorph.9 | 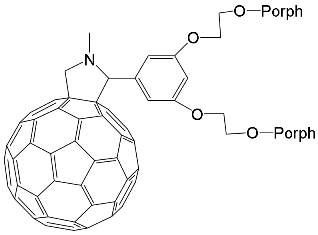 |
DABCO | [72] |
| ZnPorph.13 | 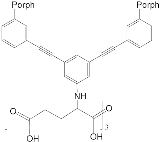 |
 |
[73] | ZnPorph.14 | 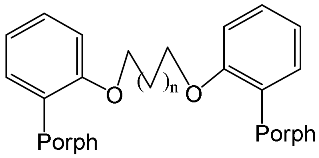 |
 |
[74] |
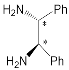
| Bis-porphyrin “molecular tweezers” for recognition of chiral guest molecules. | |||||||
| ZnPorph.9 | 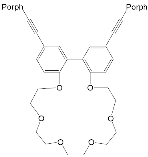 |
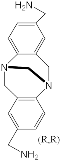 |
[75] | ZnPorph.9 | 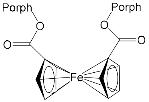 |
 |
[76] |
| ZnPorph.15 MgPorph.15 |
 |
 |
[15] | ZnPorph.15 MgPorph.15 |
 |
 |
[15] |
| ZnPorph.14 |  |
 |
[15] | ZnPorph.9 |  |
 |
[15] |
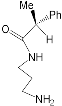
| MPorph. | R5, R10,R15 | R20 | R2,3,7,8,12,13,17,18 | MPorph. | R5, R10,R15 | R20 | R2,3,7,8,12,13,17,18 | 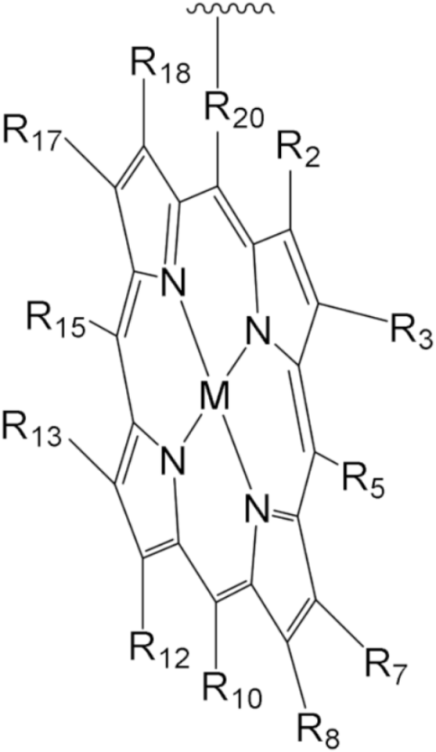 |
| 1 | 4-Me-Ph | Ph | H | |||||
| 2 | 4-Me-Ph | – | 9 | Ph | H | |||
| 3 | 3,5-But-Ph | Ph | 10 | H | – | 2,3,7,13,17,18-H, 8,12-n-C5H14 | ||
| 4 | (2,3,4,5,6-F-Ph) | 11 | Et | |||||
| 5 | 2,4,6-Me-Ph | 13 | 4-COOH-Ph | Ph | H | |||
| 6 | 5,15-H;10-(3,5But-Ph) | 2,8,12,18-Me, 3,7,13,17-Bu | 12 | 5,10-Py, 15-Ph | ||||
| 7 | 2,15-H, 10-Ph | – | 2,8,12,18-Me,3,7,13,17-Et | 14 | Ph | – | ||
| 8 | H | 2,3,17,18-H, 7,8,12,13-Et | 15 | H | 2,8,12,18-Me, 3,7,13,17-Et | |||
The 1H-NMR spectra of porphyrinate 7 in the presence of pyridine (Py) and imidazole (Im) have two rows of signals of aromatic protons belonging to the free and bound porphyrinate molecules of the substrate ( Figure 1 ). The ring current shielding effect of the porphyrin macrocycle results in an up-field shift of the aromatic protons of the bound substrate molecules compared with the respective protons of the unbound substrate molecules.
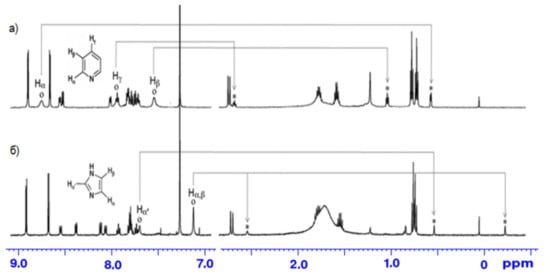
Thus, Zn-porphyrin 7 capped with calix[4]arene offers in its cavity a guest-binding environment for selective binding of small organic molecules, which makes it possible to distinguish even small structural differences between pyridine and 4-methylpyridine. Guests that are shaped in a way that is complementary to the confined cavity are capable of fitting into and binding to the cavity by van der Waals attractive interactions.
The synthesis and binding ability of a Zn-porphyrin ( 12) with a calix[5]arene cap were presented in [49]. In order to synthesize such a bridged porphyrin, 5,15-Bis-(carboxynaphtyl)-10,20-diphenylporphyrin ( 13) with syn arrangement of two naphthalene rings was prepared. Reaction of the porphyrin 13 with calix[5]arene with two amino functions ( 14) and subsequent treatment of the reaction product with zinc acetate gave the calix[5]arene-capped Zn-porphyrin 12 ( Scheme 4 ).
3. Molecular Receptors Based on Porphyrins Modified at the Macrocycle Periphery with Bulky Substituents
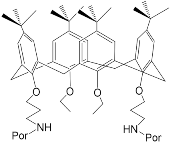
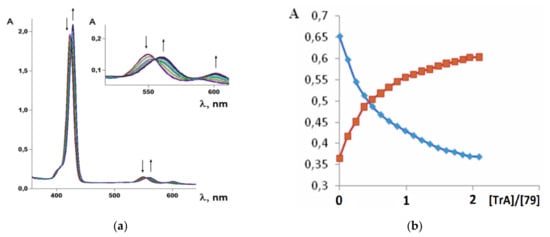
It was established by the spectrophotometric titration and 1H-NMR methods that the formation of a complex between zinc porphyrinate ( 79) and triazole (TrA) ( Scheme 26 ) leads to the formation of one family of spectral curves corresponding to its own set of isobestic points ( Figure 18 a).
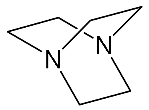
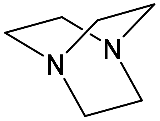
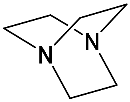
It should be said that complex formation is accompanied by considerable changes in the cryptand conformation, according to [111]. Figure 23 shows an example of complex formation between cryptand-[2,2,2] and a potassium cation and conformational changes accompanying this process in the complex.
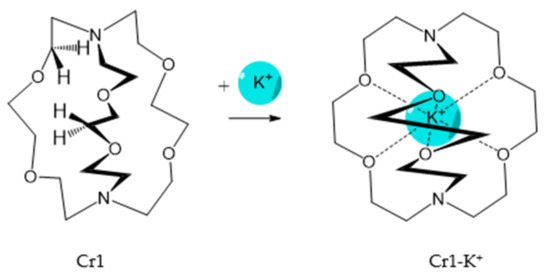
It should be also said that the 79-Cr1 complex is destroyed as well when trifluoroacetic acid is added to the solution ( Scheme 30 ). If the porphyrinate–ligand complex destruction under the action of the metal cation is caused by changes in the cryptate geometry in comparison with that of the cryptand, the complex destruction under the action of the acid is, evidently, caused by the breakage of the zinc–nitrogen bond between the porphyrinate metal cation and cryptand nitrogen upon protonation of the cryptand nitrogen atom.
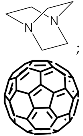
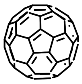
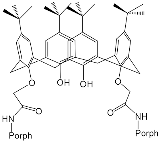
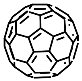
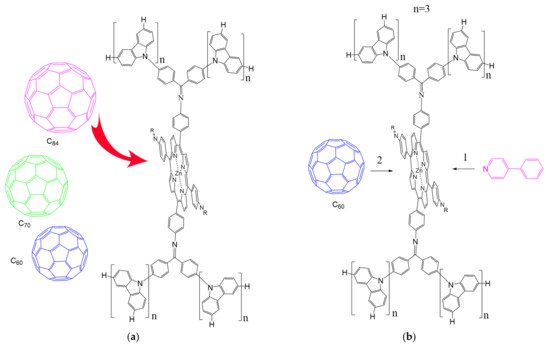
According to [113], the formation of a complex between the porphyrinate zinc and the phenyl-pyridine nitrogen atom ( Figure 29 ) causing conformational changes in the macrocycle dendrimer environment affects the ability of the outer carbazole layer of the 87G 2 receptor to form complexes with C 60.
This entry is adapted from the peer-reviewed paper 10.3390/molecules26175292
