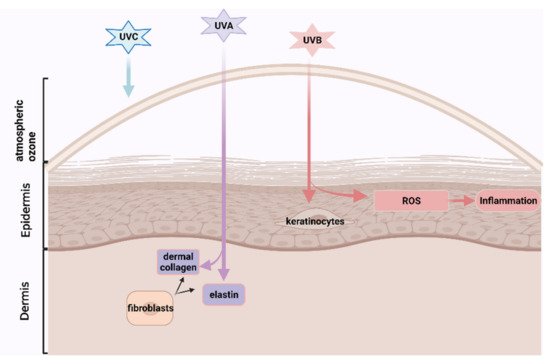
Skin ageing is becoming a global challenge due to longer human longevity and intensive ultra-violet rays contributed to the ozone layer destruction, attracting greater scientific interests in developing nutricoesmetic products, especially from natural functional ingredients with lower side-effects. Astaxanthin, a carotenoid with high antioxidant and anti-inflammatory effects, which could be extracted from the byproduct of processed crustaceans, prevented skin photoaging and age-related skin diseases in the animal models. But these byproducts are generally discarded as marine waste, losing potentially valuable ingredients, and causing serious environmental problems by accumulating high amounts of organic materials. ASX may have cosmetical potential to prevent or reverse human skin ageing, which has been evaluated in several previous papers. Here, we conducted a systemic review and meta-analysis to help clarify its human skincare effects, which promote marine waste recycling, environmental protection, and sustainable development.
- astaxanthin
- skin ageing
- antioxidant
- anti-inflammation
1. Introduction
2. Method
To conduct a comprehensive systematic literature search, the controlled vocabulary and free text terms and Boolean operators “AND” and “OR” were used. The following text words: ("astaxanthine”OR“astaxanthine”OR“astaxanthin”OR “astaxanthins” OR“ASX” OR “carotenoid”) AND (“skin” OR “skin derm *”) appeared in all fields of the articles. Multiple databases, including PubMed, Scopus, and Web of Science, were searched, yielding articles published in English between 2001 and 2021.
A study was included if it met all of the inclusion criteria, and a study was excluded if it met one or more of the exclusion criteria.
The following outcomes were captured: moisture content, sebum content, skin elasticity, TEWL, wrinkle depth, sebum oil, presence of oxidative stress biomarkers (thymine dimers and 8-hydroxy-2 0 -deoxyguanosine (8-OHdG), malondialdehyde (MDA)), mRNA expressions of procollagen type I and fibrillin-1, Matrix Metalloproteinase (MMP)-1, and MMP-2, interleukin (IL)-1 α, minimal erythema dose (MED), mean depth of texture, the total area of corneocyte, inflammatory biomarkers (C-reactive protein (CRP)), lipid droplet size and corneocytes desquamation. Some of the selected studies performed outcome measurements at several key points during study periods, the effects of the longest durations (endpoints) were extracted. However, the baseline and endpoint measure (mean value) and its variability (either standard deviation or standard error of the mean) of some outcomes were presented in the figures and graphs. They were then extracted by applying GetData Graph Digitizer [19].
The randomised, controlled studies (RCTs) were grouped according to the similarity of each outcome related to skin ageing, and meta-analysis was conducted for each group independently using Review Manager 5.4 [20]. And the statistical analysis used the random-effect model to calculate the mean difference and standard deviations (SDs) for continuous variables. Although the included RCTs have assessed several skin ageing parameters, meta-analysis was conducted only if there were at least 3 available identified studies for each parameter.
Furthermore, Higgins I (I^2) represents the level of heterogeneity among the included studies and measures the proportion of inconsistency that cannot be explained by chance alone [21], ranging from 0% to 100%. I^2 < 25% indicated very low, 25% < I^2 < 50% reported low heterogeneity, 50% < I^2 < 75% indicated moderate heterogeneity, whereas I^2 ≥ 75% corresponded to substantial-high heterogeneity [22]. If there was a small or absent heterogeneity, a fixed-effects model was used in the meta-analysis [22]. As included studies did not apply the same outcome measure and scales, the standardized mean difference was proposed to be used [22].
The probability (p) value of ≤0.05 was regarded as statistically significant. The result of the overall effect test was shown as a standardized mean difference and 95% confidence interval (CI). The small effect sizes were 0.2 and below, the medium size ranged from 0.3 to 0.7 and the large effect size were 0.8 and above.
The methodological quality of included studies was systematically assessed using Cochrane Collaboration's tool for evaluating the risk of bias (RoB) [23], which involves seven items, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. Following the guidelines in the Cochrane handbook, there are three options for judgments: low risk of bias in green, unclear risk of bias in yellow, and high risk of bias in red, respectively [24].
Furthermore, the results for topical, oral-topical ASX administrations on skin ageing from open-label, prospective studies, and extracted skin parameters from RCTs which were not pooled into meta-analyses, would be summarized in percentage, calculated by: Improvement from baseline = ((endpoint mean − baseline mean) ÷ baseline mean) × 100%.
3. Development and Findings
The literature search finally identified 11 full-text publications. Eight randomised controlled studies (RCTs) were pooled into a meta-analysis to assess the effects of oral ASX capsules on skin ageing, and one RCT provided additional information about the antioxidants and anti-inflammatory properties of ASX[25]. There were three open-label, prospective trials [13][26][27] to evaluate topical, oral-topical applications of ASX on skin ageing. Tominaga et al. (2012) [28] and Seki et al. (2001) [26] conducted two clinical trials in the same article. One open-label, prospective study assessed the effect of oral ASX capsules intake on influential factors of epidermal barrier functions[27].
The eight RCTs involved 162 subjects in the ASX supplement group and 131 subjects in the placebo group. They were initially divided into three groups according to the measured outcomes, including moisture content, skin elasticity and wrinkle depth and then subjected to the meta-analysis.
Figure 3A displays the forest plot of the meta-analysis of five studies with respect to combined moisture content estimates comparing the placebo group with the group of participants orally supplemented with ASX. For this outcome, supplementation resulted in statistically significant improvement (Z = 2.17, p = 0.03), as based on the overall effect size of 0.53 (95% CI = 0.05, 1.01).
In the meta-analysis with 6 trials (Figure 3B) that evaluated skin elasticity, oral ASX supplementation also significantly improved the outcome (Z = 2.61, p = 0.009) in comparison to the placebo group, with an overall effect size of 0.77 (95% CI = 0.19, 1.35).
No significant differences (Z = 1.58, p = 0.11) were identified in studies between the ASX treatment and placebo groups in studies evaluating the wrinkle depth. Figure 3C shows a general effect size of −0.26 (95% CI = −0.58, 0.06).
For other extracted skin parameters, sebum content and sebum oil, which was reported in a single study, as well as TEWL which were reported by four trials without enough data, were not pooled into meta-analysis.
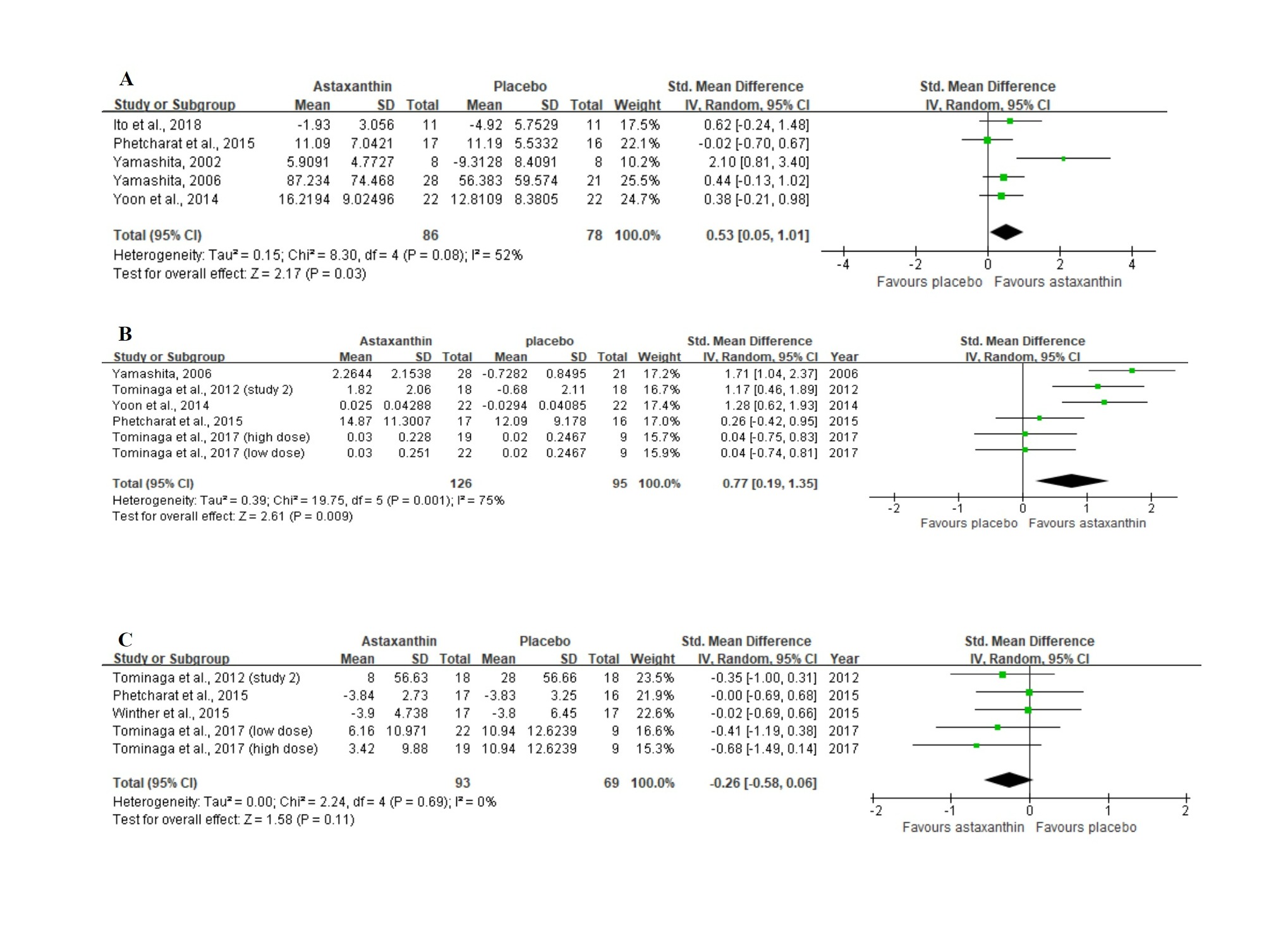 Figure 3. Forest plots summarizing the impact of ASX oral supplementation on (A) the increase in moisture content (µS), and (B) the increase in skin elasticity (%) using a random-effects model, as well as (C) the reduction in wrinkle depth (µm) using a fixed-effects model. Pooled summary data are presented as mean differences compared to control. The area of each green symbol is proportional to the weight of the study. Squares represent the standardized mean differences, bars represent the 95% CI, and diamonds show the pooled effect.
Figure 3. Forest plots summarizing the impact of ASX oral supplementation on (A) the increase in moisture content (µS), and (B) the increase in skin elasticity (%) using a random-effects model, as well as (C) the reduction in wrinkle depth (µm) using a fixed-effects model. Pooled summary data are presented as mean differences compared to control. The area of each green symbol is proportional to the weight of the study. Squares represent the standardized mean differences, bars represent the 95% CI, and diamonds show the pooled effect.
TEWL was reduced from baseline by 49.52% after administrating with 2 mg/d of ASX and 3 mg/d of collagen hydrolysate for 12 weeks while there was a 36.96% of TEWL reduction in the control group [9]. It is consistent with another study that six weeks of 6 mg/d of ASX supplementation resulted in a significant decrease in TEWL from the baseline while there was an increase in TEWL in the control group (study 2) [28].
Sebum content of the cheek has been maintained by four weeks of oral ASX and tocotrienol intake from baseline, while sebum content was decreased by 7.21 (without unit) in the placebo group at the end of the study [29]. A substantial reduction in sebum oil of cheek from baseline was observed after six weeks of applications of 6 mg/d of ASX oral supplementation compared to the placebo group [28].
Two open-label prospective studies measured the effects of topical and oral-topical ASX applications on skin ageing.
The moisture content of the left outer canthus was significantly increased from baseline by 3.32% after three week-topical administration of 280 mg/d of ASX cream (study 1) [26]. The improvements in skin conditions such as dryness, flushing, itching and inconsistency with makeup were observed from questionnaire responses after three weeks of repeated ASX topical applications (study 1) [26]. The second study by Seki et al. (2001) [26] also showed a 106.67% elevation in skin moisture content of left outer canthus after a two-week application of 140 mg/d of ASX-based cream in participants with dried and mixed skin. An antiwrinkle effect of ASX under eyes and outer canthus was observed in three subjects with different skin types by using magnified photographs. Skin conditions including moistness, smoothness, and elasticity obtained from skin-palpation and skin inspection by beauty specialists, were improved in all three subjects.
Significant wrinkle reductions and elasticity improvements were observed at outer canthus by 2.27% and 3.39%, respectively, after eight weeks of applications with 6 mg/d of oral ASX capsules and 94.18 mg/d of topical ASX cream [28]. A combination of ASX techniques showed a significant increase in moisture content of the corneocyte layer, the total area of the corneocyte, and the mean depth of skin texture in the cheek in 10 participants with dried skin [28].
Figure 4 shows the assessment of RoB at the domain level revealed an unclear RoB for most studies.
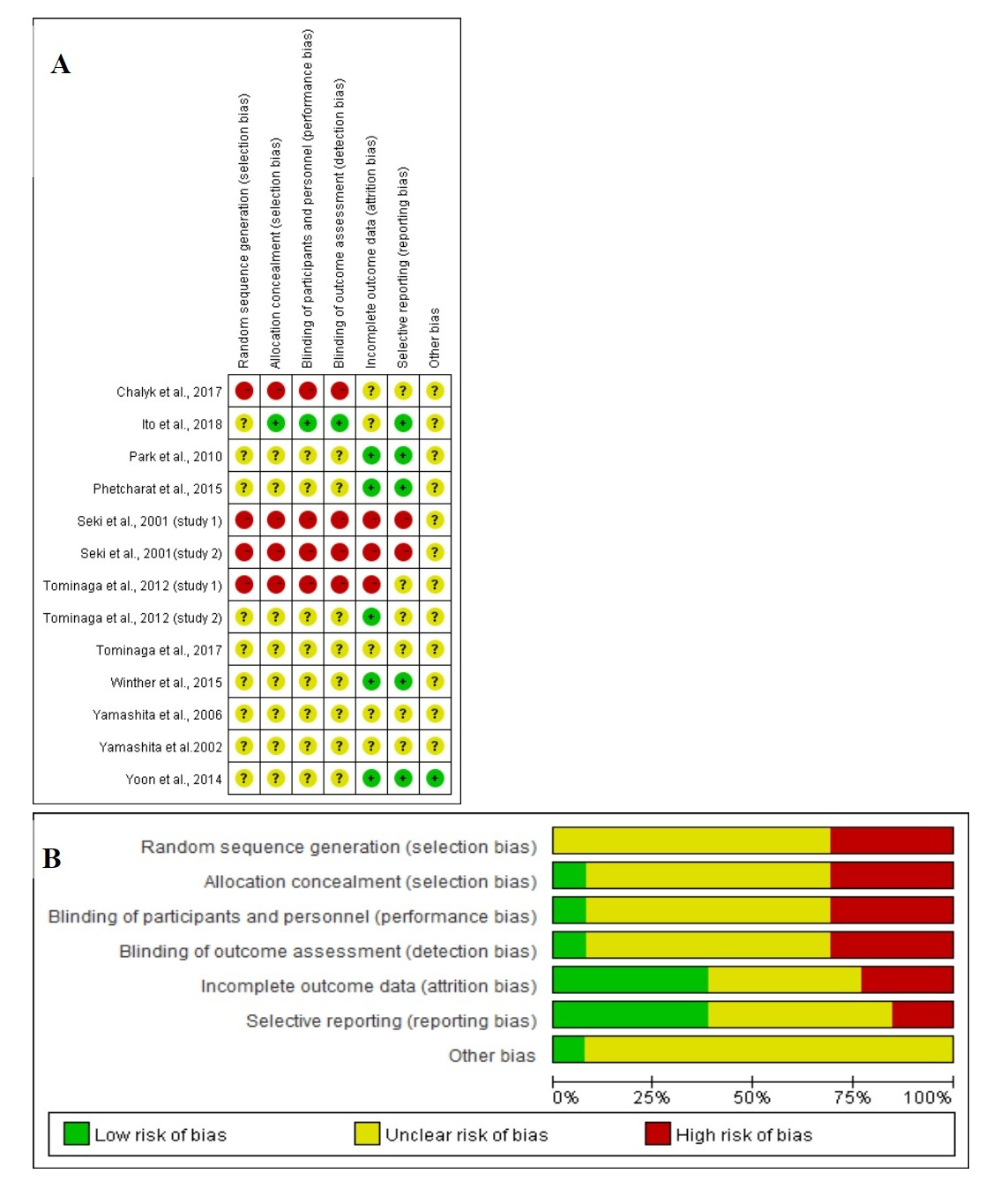
Figure 4. (A) Graph of RoB for each study according to the seven domains defined by Cochrane Collation’s tool. (B) Review of each RoB item presented as percentages across all included studies according to the judgments of the author.
4. Conclusion
Given the results of the meta-analysis and weak evidence from open-label, prospective studies, ASX oral and/or topical applications may delay and improve the signs of skin ageing by enhancing moisture content and skin elasticity, reducing facial wrinkles and sebum oil due to its antioxidant, anti-inflammatory effects and improved effects on skin barrier integrity. Oral supplementations might be more sustained and pronounced than topical applications. A synergistic skin protective effect was found in the combinational usages.
This systemic review could only partially agree with previous narrative reviews which reported the ASX oral applications probably prevented skin ageing in healthy middle-aged participants with defined age-related signs at baseline [2][14][30]. Because the reliability and strength of the evidence in this review were limited by small sample sizes, imperfect study design, method of data extraction and potential conflicts of interests, resulting in unclear RoB. It is thus necessary for future studies to design more large-scale randomized, blinded, controlled trials by recruiting more middle-aged healthy female and male participants across various regions especially for people from Western countries, using more objective dermatological assessments and reporting the original data.
This entry is adapted from the peer-reviewed paper 10.3390/nu13092917
References
- Kuhn, R. The coloring matters of the lobster (Astacus gammarus L.). Z. Angew. Chem. 1938, 51, 465–466.
- Ng, Q.X.; De Deyn, M.L.Z.Q.; Loke, W.; Foo, N.X.; Chan, H.W.; Yeo, W.S. Effects of astaxanthin supplementation on skin health: A systematic review of clinical studies. J. Diet. Suppl. 2021, 18, 169–182.
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196.
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern. Med. Rev. 2011, 16, 355–364.
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Burdeos, G.C.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571.
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive effect of dietary astaxanthin on UVA-induced skin photoaging in hairless mice. PLoS ONE 2017, 12, e0171178.
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208.
- Natsuga, K. Epidermal barriers. Cold Spring Harb. Perspect. Med. 2014, 4, a018218.
- Yoon, H.-S.; Cho, H.H.; Cho, S.; Lee, S.-R.; Shin, M.-H.; Chung, J.H. Supplementing with dietary astaxanthin combined with collagen hydrolysate improves facial elasticity and decreases matrix metalloproteinase-1 and-12 expression: A comparative study with placebo. J. Med. Food 2014, 17, 810–816.
- Gilchrest, B.; Yaar, M. Ageing and photoageing of the skin: Observations at the cellular and molecular level. Br. J. Dermatol. 1992, 127, 25–30.
- Tyrrell, R.M. Activation of mammalian gene expression by the UV component of sunlight—From models to reality. Bioessays 1996, 18, 139–148.
- Kuwazuru, O.; Miyamoto, K.; Yoshikawa, N.; Imayama, S. Skin wrinkling morphology changes suddenly in the early 30s. Ski. Res. Technol. 2012, 18, 495–503.
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 17–35.
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522.
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38.
- Yoshihisa, Y.; Andoh, T.; Matsunaga, K.; Rehman, M.U.; Maoka, T.; Shimizu, T. Efficacy of astaxanthin for the treatment of atopic dermatitis in a murine model. PLoS ONE 2016, 11, e0152288.
- Patruno, C.; Napolitano, M.; Argenziano, G.; Peris, K.; Ortoncelli, M.; Girolomoni, G.; Offidani, A.; Ferrucci, S.; Amoruso, G.; Rossi, M. Dupilumab therapy of atopic dermatitis of the elderly: A multicentre, real-life study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 958–964.
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-inflammatory effect of astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp. Dermatol. 2018, 27, 378–385.
- Digitizer, G.G. Getdata-Graph-Digitizer. 2020. Available online: http://getdata-graph-digitizer.com/ (accessed on 19 February 2021).
- The Nordic Cochrane Centre, T.C.C. Review Manager (RevMan). 2020. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 19 February 2021).
- Yamashita, E. Cosmetic benefit of dietary supplements including astaxanthin and tocotrienol on human skin. 2002. Food Style 2002, 21, 6.
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 2168.
- Seki, T.; Sueki, H.; Kono, H.; Suganuma, K.; Yamashita, E. Effects of astaxanthin from Haematococcus pluvialis on human skin-patch test; skin repeated application test; effect on wrinkle reduction. Fragr. J. 2001, 12, 98–103.
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011, 343, d5928.
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P.; Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab 2010, 7, 18, .
- Yamashita, E. Cosmetic benefit of dietary supplements including astaxanthin and tocotrienol on human skin. 2002. Food Style 2002, 21, 6.
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 2168.
- Seki, T.; Sueki, H.; Kono, H.; Suganuma, K.; Yamashita, E. Effects of astaxanthin from Haematococcus pluvialis on human skin-patch test; skin repeated application test; effect on wrinkle reduction. Fragr. J. 2001, 12, 98–103.
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M.; Continuous astaxanthin intake reduces oxidative stress and reverses age-related morphological changes of residual skin surface components in middle-aged volunteers. Nutr. Res. 2017, 48, 40-48, .
- Singh, K.N.; Patil, S.; Barkate, H. Protective effects of astaxanthin on skin: Recent scientific evidence, possible mechanisms, and potential indications. J. Cosmet. Dermatol. 2020, 19, 22–27.
