Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
As an apoplastic signal, extracellular ATP (eATP) promotes pollen germination (PG) and pollen tube growth (PTG) of Arabidopsis thaliana by stimulating Ca2+ or K+ absorption. P2K1 receptor, heterotrimeric G alpha protein and CNGC2/CNGC4 are involved in eATP stimulated signaling in Arabidopsis pollens.
- extracellular ATP (eATP)
- Arabidopsis thaliana
- pollen grain
- ion influx
- signaling
1. Introduction
The apoplast, including the cell wall and intercellular space, plays essential roles in modulating plant cell growth and development due to the existence of numerous signaling molecules within the apoplastic matrix. In recent years, growing evidence has shown that adenosine triphosphate (ATP) is present in the apoplast and participates in physiological processes such as vegetative growth, development, and stress response [1][2][3][4]. eATP is involved in maintaining cell viability [5] and the growth rate of cultured cells [6], regulating the rate or direction of the growth of roots [7], hypocotyls [8][9], and cotton fibers [10]. It is also involved in modulating stomatal movement [11][12][13]. Some stresses induce eATP release as a “danger signal”, which in turn induces resistance responses to disease, hypotonic conditions, high salinity, and cold stress [14][15][16][17][18].
The signal transduction of eATP has been intensively investigated over the past two decades. Two lectin receptor kinases, P2K1 and P2K2, were identified to be eATP receptors in Arabidopsis thaliana [19][20] involved in eATP-induced immune responses [13][14][21]. Signal transducers in the plasma membrane (PM), including heterotrimeric G proteins [12][22], NADPH oxidase [23][24], and Ca2+ channels [6][23][25][26], were reported to be involved in eATP signal transduction. Several secondary messengers, e.g., ROS, H2O2, NO, and Ca2+ may be responsible for the eATP-induced intracellular responses that eventually alter plant growth and development [6][17][26][27][28][29].
PG and PTG are fundamental processes in plant sexual reproduction. Ion intake is the main factor affecting the two processes [30][31][32]. Through the regulation of turgor pressure, K+ ions play essential roles in PG and PTG. Proper K+ channel expression is essential for Arabidopsis pollen hydration on stigma [33]. K+ influx causes pollen tube apex bursting and the release of sperm cells to complete double fertilization [34] and endows pollen with competitive ability [35]. Excessively low or high K+ or Ca2+ concentration inhibited PG and PTG in vitro in Arabidopsis [36][37]. Inward K+ channels that drive K+ absorption by pollen cells are involved in PG and PTG [33][38][39][40]. Ca2+ is involved in modulating the activity of some inward K+ channels in pollen protoplasts [39][41].
As a component of the cell wall and a cytoplasmic messenger, Ca2+ ions play essential roles in PG and PTG [31][32][42][43][44][45]. Sufficient Ca2+ is necessary for PG and PTG. Pollen tubes emerge from the aperture where high cytosolic Ca2+ concentration ([Ca2+]cyt) is localized [42][46][47]. [Ca2+]cyt initiates pollen germination [46] and regulates the rate and direction of growth of pollen tubes [44][45][48][49][50][51]. Exocytosis occurs continuously during PG and PTG. Ca2+-regulated vesicle trafficking and fusion with the PM therefore play key roles in PG and PTG [51][52][53][54][55][56][57]. Ca2+ influx and signaling are responsible for stimuli-triggered or -regulated PG and PTG [58][59][60][61][62][63].
2. ATP Addition Impacts PG and PTG in Pollens from 34 Species
To verify the universality of eATP-regulated PG and PTG, we examined the effects of 0.1 mM ATP on PG and PTG of pollen grains from 34 species. Pollen germination rates increased significantly in 28 species (p < 0.05) and decreased significantly in 6 species (p < 0.05). Pollen tube length measurement results showed that tube growth was significantly promoted in 19 species (p < 0.05) and significantly inhibited in 5 species, while no effect was observed in 10 species (Figure 1).
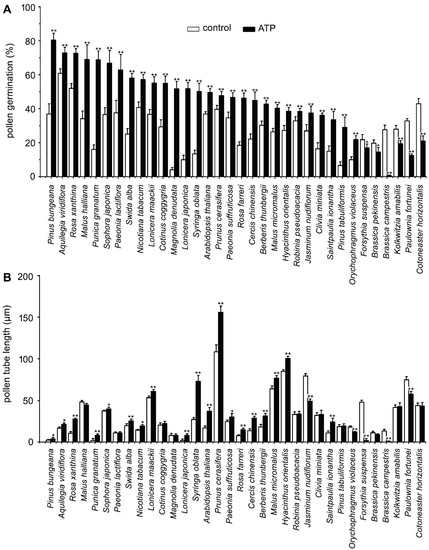
Figure 1. ATP-regulated PG and PTG in various plant species. Pollen grains of 34 plant species were germinated in vitro in the medium containing 18% sucrose, 0.1 mM KCl, and 0.5% agarose. Germination duration of each species is listed in Table 1. PG (A) and PTG (B) were noted. In each experiment, at least 300 pollen grains or 150 pollen tubes were counted or measured. Data from 3 replicates were calculated to obtain mean ± SD. Student’s t-test p values: * p < 0.05, ** p < 0.01.
Table 1. Plant materials for in vitro pollen germination (sorted by the initials of the Latin names of plant species).
| Latin Name | Family | Class | Phylum | Germination Duration (min) | |
|---|---|---|---|---|---|
| 1 | Aquilegia viridiflora | Ranunculaceae | Dicotyledoneae | Angiospermae | 30 |
| 2 | Arabidopsis thaliana | Brassicaceae | Dicotyledoneae | Angiospermae | 300 |
| 3 | Berberis thunbergii | Berberidaceae | Dicotyledoneae | Angiospermae | 40 |
| 4 | Brassica campestris | Brassicaceae | Dicotyledoneae | Angiospermae | 240 |
| 5 | Brassica pekinensis | Brassicaceae | Dicotyledoneae | Angiospermae | 150 |
| 6 | Cercis chinensis | Leguminosae | Dicotyledoneae | Angiospermae | 90 |
| 7 | Clivia miniata | Amaryllidaceae | Monocotyledoneae | Angiospermae | 420 |
| 8 | Cotinus coggygria | Anacardiaceae | Dicotyledoneae | Angiospermae | 40 |
| 9 | Cotoneaster horizontalis | Rosaceae | Dicotyledoneae | Angiospermae | 60 |
| 10 | Forsythia suspensa | Oleaceae | Dicotyledoneae | Angiospermae | 120 |
| 11 | Hyacinthus orientalis | Hyacinthaceae | Monocotyledoneae | Angiospermae | 80 |
| 12 | Jasminum nudiflorum | Oleaceae | Dicotyledoneae | Angiospermae | 90 |
| 13 | Kolkwitzia amabilis | Caprifoliaceae | Dicotyledoneae | Angiospermae | 60 |
| 14 | Lonicera maackii | Caprifoliaceae | Dicotyledoneae | Angiospermae | 60 |
| 15 | Lonicera japonica | Caprifoliaceae | Dicotyledoneae | Angiospermae | 120 |
| 16 | Magnolia denudata | Magnoliaceae | Dicotyledoneae | Angiospermae | 2880 |
| 17 | Malus halliana | Rosaceae | Dicotyledoneae | Angiospermae | 30 |
| 18 | Malus micromalus | Rosaceae | Dicotyledoneae | Angiospermae | 60 |
| 19 | Nicotiana tabacum | Solanaceae | Dicotyledoneae | Angiospermae | 30 |
| 20 | Orychophragmus violaceus | Brassicaceae | Dicotyledoneae | Angiospermae | 180 |
| 21 | Paeonia suffruticosa | Paeoniaceae | Dicotyledoneae | Angiospermae | 60 |
| 22 | Paeonia lactiflora | Paeoniaceae | Dicotyledoneae | Angiospermae | 60 |
| 23 | Paulownia fortunei | Scrophulariaceae | Dicotyledoneae | Angiospermae | 60 |
| 24 | Pinus bungeana | Pinaceae | Coniferopsida | Gymnosperm | 4320 |
| 25 | Pinus tabulaeformis | Pinaceae | Coniferopsida | Gymnosperm | 4320 |
| 26 | Punica granatum | Punicaceae | Dicotyledoneae | Angiospermae | 120 |
| 27 | Prunus cerasifera | Rosaceae | Dicotyledoneae | Angiospermae | 60 |
| 28 | Rosa xanthina | Rosaceae | Dicotyledoneae | Angiospermae | 20 |
| 29 | Robinia pseudoacacia | Leguminosae | Dicotyledoneae | Angiospermae | 30 |
| 30 | Rosa farreri | Rosaceae | Dicotyledoneae | Angiospermae | 30 |
| 31 | Saintpaulia ionantha | Gesneriaceae | Dicotyledoneae | Angiospermae | 180 |
| 32 | Sophora japonica | Leguminosae | Dicotyledoneae | Angiospermae | 30 |
| 33 | Swida alba | Corneceae | Dicotyledoneae | Angiospermae | 60 |
| 34 | Syringa oblata | Oleaceae | Dicotyledoneae | Angiospermae | 90 |
3. eATP Regulates PG and PTG of Arabidopsis thaliana via K+ and Ca2+ Intake
To verify the role of ion uptake in eATP-promoted PG and PTG, Arabidopsis thaliana pollen was germinated in vitro, and the effects of apyrase or ATP addition on PG and PTG were investigated. In the basic medium, which contained 0.1 mM KCl, apyrase inhibited PG and PTG significantly. After 100 units/mL apyrase treatment, the pollen germination rate decreased from 39.2 ± 2.5% to 26.7 ± 3.4%, and the pollen tube length decreased from 16.9 ± 2.4 μm to 10.3 ± 1.8 μm (p < 0.05). Control treatment with heat-denatured apyrase or bovine serum albumin (BSA) did not affect PG or PTG (p > 0.05) (Figure 2A,D). At low K+ concentrations (0.02–0.1 mM), ATP supplementation (0.1 mM) promoted PG and PTG significantly, while at high K+ concentration (1.0 mM), ATP supplementation slightly inhibited PG but did not affect PTG (Figure 2B,E). To confirm that eATP supplementation promotes PG and PTG by increasing K+ uptake, 1 mM of the K+ channel blockers Ba2+, Cs+, or TEA (tetraethylammonium) was added into the basic medium containing 0.1 mM KCl. In these conditions, the effects of eATP on PG or PTG were significantly suppressed (p < 0.01) (Figure 2C,F).
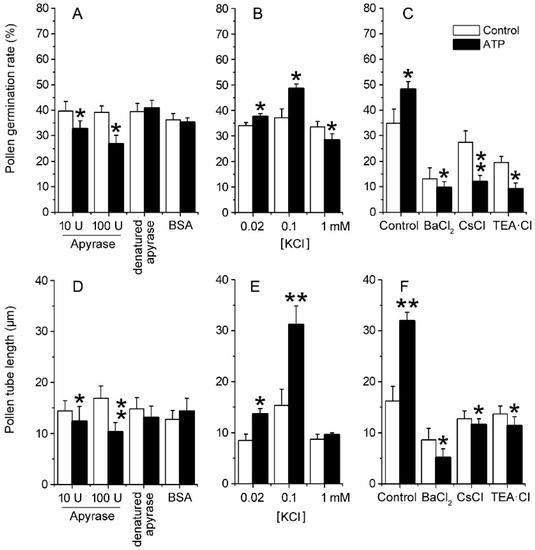
Figure 2. eATP regulates the PG and PTG of Arabidopsis thaliana by facilitating K+ uptake. Apyrase-inhibited PG (A) and PTG (D), ATP-regulated PG (B), and PTG (E) in medium containing serial concentrations of KCl. K+ channel blockers (1 mM) inhibited ATP-promoted PG (C) and PTG (F). In (A–F), the K+ concentration in the medium is 0.1 mM. In each experiment, at least 300 pollen grains were counted to obtain PG, and at least 150 pollen tubes were measured to obtain the pollen tube length. Data from 3 replicates were combined to obtain the mean ± SD. Student’s t test p values: * p < 0.05, ** p < 0.01.
To verify the role of Ca2+ in eATP-promoted PG and PTG, 0.1 mM ATP was added to medium containing EGTA, series of concentrations of Ca2+, or Ca2+ channel blockers. In 0.5 mM EGTA-containing medium, PG and PTG were significantly suppressed, and ATP supplementation did not have any effect. In medium containing 0.1 mM CaCl2, PG and PTG were both stimulated by 0.1 mM ATP. In medium containing 1 mM CaCl2, pollen tube growth was markedly stimulated, while pollen germination was suppressed by 0.1 mM ATP. In medium containing 0.1 mM Ca2+-permeable channel blockers (Gd3+ or La3+), resting PG and PTG were both suppressed, and added ATP did not have any effect on PG and PTG (Figure 3). These data indicate that eATP may promote PG and PTG by facilitating Ca2+ intake.
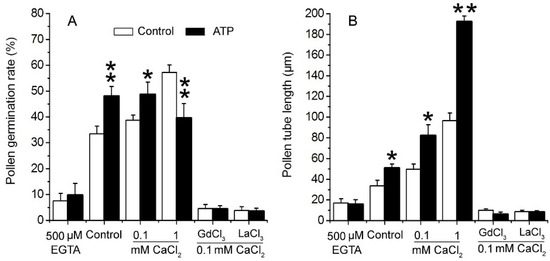
Figure 3. eATP regulates PG and PTG of Arabidopsis thaliana by facilitating Ca2+ uptake. ATP (0.1 mM) regulates PG (A) and PTG (B) in medium containing a Ca2+ chelator, serial concentrations of Ca2+, and channel blockers (0.1 mM). In each experiment, at least 300 pollen grains were counted to obtain PG, and at least 150 pollen tubes were measured to obtain the pollen tube length. Data from 3 replicates were combined to obtain the mean ± SD. Student’s t test p values: * p < 0.05, ** p < 0.01.
To verify the role of ATP hydrolytes in PG and PTG, 0.1 mM ADP, AMP, adenosine, adenine, or phosphate (Pi) was added to the medium containing 0.1 mM KCl or CaCl2. PG and PTG were not affected by these reagents in either KCl or CaCl2 medium. To verify the effect of various ATP salts on PG and PTG, 0.1 mM ATP sodium, Tris, or magnesium salt was added to the medium containing 0.1 mM KCl or CaCl2. PG and PTG were significantly promoted by the three ATP salts (p < 0.05) in both the KCl and CaCl2 media. To ensure that ATP acts as a signal rather than an energy carrier, 0.1 mM ATPγS, a weakly-hydrolyzable ATP analog, was added to the medium containing either 0.1 mM KCl or CaCl2. PG and PTG were significantly promoted by ATPγS (p < 0.05) in the KCl and CaCl2 media.
4. ATP Stimulates K+ and Ca2+ Influx in Arabidopsis thaliana Pollen Protoplast
To confirm that eATP stimulates K+ uptake in pollen grains, whole-cell patch clamping was performed to detect the effect of eATP on K+ conductance in the PM. In pollen protoplasts, a time-dependent inward-rectifying K+ current was recorded at a negative voltage of more than −140 mV. The addition of 1 mM CsCl significantly suppressed the current intensity, confirming that the inward currents may be carried by K+ influx (Figure 4A). The addition of 0.1 mM ATP stimulated the inward K+ current: the maximum current intensity increased from −129 ± 32 pA to −254 ± 34 pA at −200 mV (n = 7, p < 0.05), and the open potential shifted to more positive (Figure 4B). In CsCl-pretreated protoplasts, the effect of ATP on the inward K+ currents was markedly suppressed. The maximum current intensity decreased from −270 ± 35 pA to −83 ± 18 pA at −200 mV (n = 7, p < 0.05) (Figure 4C).
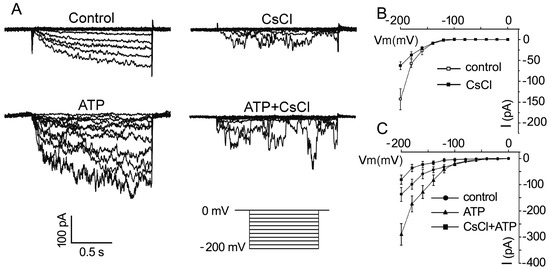
Figure 4. CsCl inhibits eATP-stimulated K+ influx in the protoplasts of Arabidopsis pollen grains. (A) CsCl (1 mM) inhibited resting and 0.1 mM ATP-stimulated inward K+ currents. Representative current traces from step-voltage clamping are shown. (B,C) I-V relationship curves of inward K+ currents (n = 7). In each experiment, data from 7 protoplasts were recorded and combined to obtain the mean ± SD.
To further confirm the promotion of eATP on the Ca2+ uptake of pollen grains, the effect of 0.1 mM ATP on inward Ca2+ conductance was investigated. The addition of 0.1 mM ATP strongly promoted Ca2+ influx in pollen protoplasts: the maximum inward current intensity at −200 mV increased from −147.6 ± 21.9 pA to −243.1 ± 22.8 pA (n = 7, p < 0.05) (Figure 5A,B). GdCl3 (1 mM) significantly suppressed Ca2+ conductance (Figure 5A,C, n = 7, p < 0.05). In Gd3+-pretreated pollen protoplasts, ATP did not stimulate Ca2+ inward conductance (Figure 5A,C, n = 7, p > 0.05).
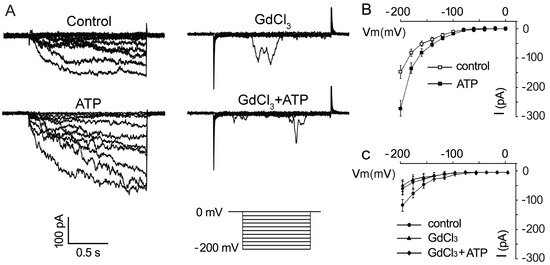
Figure 5. GdCl3 inhibits eATP-stimulated Ca2+ influx in the protoplasts of Arabidopsis pollen grain. (A) GdCl3 (0.1 mM) inhibited resting and 0.1 mM ATP-stimulated inward Ca2+ currents. Representative current traces from step-voltage clamping are shown. (B,C) I-V relationship curves of the inward Ca2+ currents (n = 7). In each experiment, data from 7 protoplasts were recorded and combined to obtain the mean ± SD.
This entry is adapted from the peer-reviewed paper 10.3390/plants10081743
References
- Cao, Y.; Tanaka, K.; Nguyen, C.T.; Stacey, G. Extracellular ATP is a central signaling molecule in plant stress responses. Curr. Opin. Plant Biol. 2014, 20, 82–87.
- Tanaka, K.; Choi, J.; Cao, Y.; Stacey, G. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front. Plant Sci. 2014, 5, 446.
- Clark, G.; Roux, S.J. Role of Ca2+ in mediating plant responses to extracellular ATP and ADP. Int. J. Mol. Sci. 2018, 19, 3590.
- Pietrowska-Borek, M.; Dobrogojski, J.; Sobieszczuk-Nowicka, E.; Borek, S. New insight into plant signaling: Extracellular ATP and uncommon nucleotides. Cells 2020, 9, 345.
- Chivasa, S.; Ndimba, B.K.; Simon, W.J.; Lindsey, K.; Slabas, A.R. Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. Plant Cell 2005, 17, 3019–3034.
- Wu, S.J.; Liu, Y.S.; Wu, J.Y. The signaling role of extracellular ATP and its dependence on Ca2+ flux in elicitation of Salvia miltiorrhiza hairy root cultures. Plant Cell Physiol. 2008, 49, 617–624.
- Kim, S.Y.; Sivaguru, M.; Stacey, G. Extracellular ATP in plants. Visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiol. 2006, 142, 984–992.
- Tonon, C.; Cecilia Terrile, M.; Jose Iglesias, M.; Lamattina, L.; Casalongue, C. Extracellular ATP, nitric oxide and superoxide act coordinately to regulate hypocotyl growth in etiolated Arabidopsis seedlings. J. Plant Physiol. 2010, 167, 540–546.
- Zhu, R.; Dong, X.; Xue, Y.; Xu, J.; Zhang, A.; Feng, M.; Zhao, Q.; Xia, S.; Yin, Y.; He, S.; et al. Redox-Responsive Transcription Factor 1 (RRFT1) is involved in extracellular ATP-regulated Arabidopsis thaliana seedling growth. Plant Cell Physiol. 2020, 61, 685–698.
- Clark, G.; Torres, J.; Finlayson, S.; Guan, X.; Handley, C.; Lee, J.; Kays, J.E.; Chen, Z.J.; Roux, S.J. Apyrase (nucleoside triphosphate-diphosphohydrolase) and extracellular nucleotides regulate cotton fiber elongation in cultured ovules. Plant Physiol. 2010, 152, 1073–1083.
- Clark, G.; Fraley, D.; Steinebrunner, I.; Cervantes, A.; Onyirimba, J.; Liu, A.; Torres, J.; Tang, W.; Kim, J.; Roux, S.J. Extracellular nucleotides and apyrases regulate stomatal aperture in Arabidopsis. Plant Physiol. 2011, 156, 1740–1753.
- Hao, L.H.; Wang, W.X.; Chen, C.; Wang, Y.F.; Liu, T.; Li, X.; Shang, Z.L. Extracellular ATP promotes stomatal opening of Arabidopsis thaliana through heterotrimeric G protein α subunit and reactive oxygen species. Mol. Plant 2012, 5, 852–864.
- Chen, D.; Cao, Y.; Li, H.; Kim, D.; Ahsan, N.; Thelen, J.; Stacey, G. Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture. Nat. Commun. 2017, 8, 2265.
- Choi, J.; Tanaka, K.; Liang, Y.; Cao, Y.; Lee, S.Y.; Stacey, G. Extracellular ATP, a danger signal, is recognized by DORN1 in Arabidopsis. Biochem. J. 2014, 463, 429–437.
- Chivasa, S.; Murphy, A.M.; Hamilton, J.M.; Lindsey, K.; Carr, J.P.; Slabas, A.R. Extracellular ATP is a regulator of pathogen defence in plants. Plant J. 2009, 60, 436–448.
- Kim, S.H.; Yang, S.H.; Kim, T.J.; Han, J.S.; Suh, J.W. Hypertonic stress increased extracellular ATP levels and the expression of stress-responsive genes in Arabidopsis thaliana seedlings. Biosci. Biotechnol. Biochem. 2009, 73, 1252–1256.
- Sun, J.; Zhang, X.; Deng, S.; Zhang, C.; Wang, M.; Ding, M.; Zhao, R.; Shen, X.; Zhou, X.; Lu, C.; et al. Extracellular ATP signaling is mediated by H2O2 and cytosolic Ca2+ in the salt response of Populus euphratica cells. PLoS ONE 2012, 7, e53136.
- Deng, S.; Sun, J.; Zhao, R.; Ding, M.; Zhang, Y.; Sun, Y.; Wang, W.; Tan, Y.; Liu, D.; Ma, X.; et al. Populus euphratica APYRASE2 enhances cold tolerance by modulating vesicular trafficking and extracellular ATP in Arabidopsis plants. Plant Physiol. 2015, 169, 530–548.
- Choi, J.; Tanaka, K.; Cao, Y.; Qi, Y.; Qiu, J.; Liang, Y.; Lee, S.Y.; Stacey, G. Identification of a plant receptor for extracellular ATP. Science 2014, 343, 290–294.
- Pham, A.Q.; Cho, S.H.; Nguyen, C.T.; Stacey, G. Arabidopsis lectin receptor kinase P2K2 is a second plant receptor for extracellular ATP and contributes to innate immunity. Plant Physiol. 2020, 183, 1364–1375.
- Chen, D.; Hao, F.; Mu, H.; Ahsan, N.; Thelen, J.J.; Stacey, G. S-acylation of P2K1 mediates extracellular ATP-induced immune signaling in Arabidopsis. Nat. Commun. 2021, 12, 2750.
- Zhu, R.; Dong, X.; Hao, W.; Gao, W.; Zhang, W.; Xia, S.; Liu, T.; Shang, Z. Heterotrimeric G protein-regulated Ca2+ influx and PIN2 asymmetric distribution are involved in Arabidopsis thaliana roots’ avoidance response to extracellular ATP. Front. Plant Sci. 2017, 8, 1522.
- Demidchik, V.; Shang, Z.; Shin, R.; Thompson, E.; Rubio, L.; Laohavisit, A.; Mortimer, J.C.; Chivasa, S.; Slabas, A.R.; Glover, B.J.; et al. Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J. 2009, 58, 903–913.
- Song, C.J.; Steinebrunner, I.; Wang, X.; Stout, S.C.; Roux, S.J. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006, 140, 1222–1232.
- Shang, Z.; Laohavisit, A.; Davies, J.M. Extracellular ATP activates an Arabidopsis plasma membrane Ca2+-permeable conductance. Plant Signal. Behav. 2009, 4, 989–991.
- Wang, L.M.; Stacey, G.; Leblanc-Fournier, N.; Legue, V.; Moulia, B.; Davies, J.M. Early extracellular ATP signaling in Arabidopsis root epidermis: A multi-conductance process. Front. Plant Sci. 2019, 10, 1064.
- Demidchik, V.; Nichols, C.; Oliynyk, M.; Dark, A.; Glover, B.J.; Davies, J.M. Is ATP a signaling agent in plants? Plant Physiol. 2003, 133, 456–461.
- Foresi, N.P.; Laxalt, A.M.; Tonon, C.V.; Casalongue, C.A.; Lamattina, L. Extracellular ATP induces nitric oxide production in tomato cell suspensions. Plant Physiol. 2007, 145, 589–592.
- Matthus, E.; Sun, J.; Wang, L.M.; Bhat, M.G.; Mohammad-Sidik, A.B.; Wilkins, K.A.; Leblanc-Fournier, N.; Legue, V.; Moulia, B.; Stacey, G.; et al. DORN1/P2K1 and purino-calcium signalling in plants: Making waves with extracellular ATP. Ann. Bot. 2019, 124, 1227–1242.
- Holdaway-Clarke, T.L.; Hepler, P.K. Control of pollen tube growth: Role of ion gradients and fluxes. New Phytol. 2003, 159, 539–563.
- Steinhorst, L.; Kudla, J. Calcium—A central regulator of pollen germination and tube growth. Biochim. Biophys. Acta 2013, 1833, 1573–1581.
- Zheng, R.H.; Su, S.; Xiao, H.; Tian, H.Q. Calcium: A critical factor in pollen germination and tube elongation. Int. J. Mol. Sci. 2019, 20, 420.
- Li, D.D.; Guan, H.; Li, F.; Liu, C.Z.; Dong, Y.X.; Zhang, X.S.; Gao, X.Q. Arabidopsis shaker pollen inward K+ channel SPIK functions in SnRK1 complex-regulated pollen hydration on the stigma. J. Integr. Plant. Biol. 2017, 59, 604–611.
- Amien, S.; Kliwer, I.; Marton, M.L.; Debener, T.; Geiger, D.; Becker, D.; Dresselhaus, T. Defensin-like ZmES4 mediates pollen tube burst in maize via opening of the potassium channel KZM1. PLoS Biol. 2010, 8, e1000388.
- Mouline, K.; Very, A.A.; Gaymard, F.; Boucherez, J.; Pilot, G.; Devic, M.; Bouchez, D.; Thibaud, J.B.; Sentenac, H. Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev. 2002, 16, 339–350.
- Fan, L.M.; Wang, Y.F.; Wang, H.; Wu, W.H. In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. J. Exp. Bot. 2001, 52, 1603–1614.
- Liu, L.; Zheng, C.; Kuang, B.; Wei, L.; Yan, L.; Wang, T. Receptor-like kinase RUPO interacts with potassium transporters to regulate pollen tube growth and integrity in rice. PLoS Genet. 2016, 12, e1006085.
- Fan, L.M.; Wu, W.H.; Yang, H.Y. Identification and characterization of the inward K+ channel in the plasma membrane of Brassica pollen protoplasts. Plant Cell Physiol. 1999, 40, 859–865.
- Zhao, L.N.; Shen, L.K.; Zhang, W.Z.; Zhang, W.; Wang, Y.; Wu, W.H. Ca2+-dependent protein kinase11 and 24 modulate the activity of the inward rectifying K+ channels in Arabidopsis pollen tubes. Plant Cell 2013, 25, 649–661.
- Yang, F.; Wang, T.; Liu, L. Pollen germination is impaired by disruption of a Shaker K+ channel OsAKT1.2 in rice. J. Plant Physiol. 2020, 248, 153140.
- Wang, S.S.; Diao, W.Z.; Yang, X.; Qiao, Z.; Wang, M.; Acharya, B.R.; Zhang, W. Arabidopsis thaliana CML25 mediates the Ca2+ regulation of K+ transmembrane trafficking during pollen germination and tube elongation. Plant Cell Environ. 2015, 38, 2372–2386.
- Pierson, E.S.; Miller, D.D.; Callaham, D.A.; Shipley, A.M.; Rivers, B.A.; Cresti, M.; Hepler, P.K. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA-type buffers and hypertonic media. Plant Cell 1994, 6, 1815–1828.
- Malho, R.; Trewavas, A.J. Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell 1996, 8, 1935–1949.
- Cole, R.A.; Fowler, J.E. Polarized growth: Maintaining focus on the tip. Curr. Opin. Plant Biol. 2006, 9, 579–588.
- Iwano, M.; Entani, T.; Shiba, H.; Kakita, M.; Nagai, T.; Mizuno, H.; Miyawaki, A.; Shoji, T.; Kubo, K.; Isogai, A.; et al. Fine-tuning of the cytoplasmic Ca2+ concentration is essential for pollen tube growth. Plant Physiol. 2009, 150, 1322–1334.
- Iwano, M.; Shiba, H.; Miwa, T.; Che, F.S.; Takayama, S.; Nagai, T.; Miyawaki, A.; Isogai, A. Ca2+ dynamics in a pollen grain and papilla cell during pollination of Arabidopsis. Plant Physiol. 2004, 136, 3562–3571.
- Rathore, K.S.; Cork, R.J.; Robinson, K.R. A cytoplasmic gradient of Ca2+ is correlated with the growth of lily pollen tubes. Dev. Biol. 1991, 148, 612–619.
- Konrad, K.R.; Wudick, M.M.; Feijo, J.A. Calcium regulation of tip growth: New genes for old mechanisms. Curr. Opin. Plant Biol. 2011, 14, 721–730.
- Kaya, H.; Nakajima, R.; Iwano, M.; Kanaoka, M.M.; Kimura, S.; Takeda, S.; Kawarazaki, T.; Senzaki, E.; Hamamura, Y.; Higashiyama, T.; et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell 2014, 26, 1069–1080.
- Parrotta, L.; Faleri, C.; Guerriero, G.; Cai, G. Cold stress affects cell wall deposition and growth pattern in tobacco pollen tubes. Plant Sci. 2019, 283, 329–342.
- Ruan, H.Q.; Li, J.; Wang, T.; Ren, H.Y. Secretory vesicles targeted to plasma membrane during pollen germination and tube growth. Front. Cell Dev. Biol. 2021, 8, 615447.
- Battey, N.H.; James, N.C.; Greenland, A.J.; Brownlee, C. Exocytosis and endocytosis. Plant Cell 1999, 11, 643–660.
- Camacho, L.; Malho, R. Endo/exocytosis in the pollen tube apex is differentially regulated by Ca2+ and GTPases. J. Exp. Bot. 2003, 54, 83–92.
- Zonia, L. Spatial and temporal integration of signalling networks regulating pollen tube growth. J. Exp. Bot. 2010, 61, 1939–1957.
- Wang, Y.F.; Fan, L.M.; Zhang, W.Z.; Zhang, W.; Wu, W.H. Ca2+-permeable channels in the plasma membrane of arabidopsis pollen are regulated by actin microfilaments. Plant Physiol. 2004, 136, 3892–3904.
- Malho, R.; Liu, Q.; Monteiro, D.; Rato, C.; Camacho, L.; Dinis, A. Signalling pathways in pollen germination and tube growth. Protoplasma 2006, 228, 21–30.
- Wu, J.Y.; Jin, C.; Qu, H.Y.; Tao, S.T.; Xu, G.H.; Wu, J.; Wu, H.Q.; Zhang, S.L. Low temperature inhibits pollen viability by alteration of actin cytoskeleton and regulation of pollen plasma membrane ion channels in Pyrus pyrifolia. Environ. Exp. Bot. 2012, 78, 70–75.
- Dutta, R.; Robinson, K.R. Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol. 2004, 135, 1398–1406.
- Frietsch, S.; Wang, Y.F.; Sladek, C.; Poulsen, L.R.; Romanowsky, S.M.; Schroeder, J.I.; Harper, J.F. A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc. Natl. Acad. Sci. USA 2007, 104, 14531–14536.
- Michard, E.; Lima, P.T.; Borges, F.; Silva, A.C.; Portes, M.T.; Carvalho, J.E.; Gilliham, M.; Liu, L.H.; Obermeyer, G.; Feijo, J.A. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 2011, 332, 434–437.
- Wu, Y.; Xu, X.; Li, S.; Liu, T.; Ma, L.; Shang, Z. Heterotrimeric G-protein participation in Arabidopsis pollen germination through modulation of a plasmamembrane hyperpolarization-activated Ca2+-permeable channel. New Phytol. 2007, 176, 550–559.
- Wu, J.; Shang, Z.; Wu, J.; Jiang, X.; Moschou, P.N.; Sun, W.; Roubelakis-Angelakis, K.A.; Zhang, S. Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+-permeable channels and pollen tube growth. Plant J. 2010, 63, 1042–1053.
- Wu, Y.; Qin, B.; Feng, K.; Yan, R.; Kang, E.; Liu, T.; Shang, Z. Extracellular ATP promoted pollen germination and tube growth of Nicotiana tabacum through promoting K+ and Ca2+ absorption. Plant Reprod. 2018, 31, 399–410.
This entry is offline, you can click here to edit this entry!
