The surface-modified separator plays a role in improving the electrolyte wettability, homogenizing Li+flux, and strengthening the mechanical/thermal property. Due to these favorable benefits, the formation of sharp Li dendrite is efficiently suppressed and the thermal stability of battery is greatly enhanced. In this article, separator-coating materials are classified into six categories in terms of material characteristics to show how each material has different electrochemical properties. We believe that the suggested approach would become a powerful strategy to improve the performance and stability of next-generation batteries such as lithium-metal batteries.
- Li metal anode
- functional separator
- battery safety
- separator-coating materials
1. Introduction
Since the advent of commercial rechargeable batteries using the combination of graphite and LiCoO 2, secondary lithium-ion batteries (LIBs) as practical energy reservoirs have received continuous attention from industries and academics, owing to their decent energy density, long life span, and low self-discharging rate [1,2,3,4,5,6,7]. As state-of-the-art electronic devices such as electric vehicles (EVs) and portable devices become a big part of our lives, the demand for developing more advanced LIBs with high capacity, fast charging ability, and improved reliability has been accelerated to keep pace with this fast-moving trend [8]. Given the specification of the above-described advanced electronic devices and the mission to reduce greenhouse gas emission to net-zero by 2050, it is inevitable to move on from the LIB technology to more advanced battery systems such as Li metal batteries (LMBs), which can dramatically increase the energy density of the existing energy storage technology [9,10,11]. Before the commercialization of LIBs, Dr. Whittingham first developed a battery prototype using a Li metal paired with a TiS 2 electrode in the 1970s [12]. The developed Li|TiS 2 cell has an electrochemical potential of about 2.5 V, which is not high enough for achieving high specific energy density. In 1989, Moli Energy commercialized Li|MoS 2 cylindrical-type rechargeable batteries that were successful in the commercial market for a while [13]. However, owing to the frequent occurrence of safety issues such as catching fire, LMB products eventually disappeared from the market at the time.
Recently, Li metal anodes have come into the spotlight again due to the limited energy density of existing LIB technology that employ a Li-containing cathode and a carbon-based anode [10,11]. Metallic Li is considered the “holy-grail” anode, owing to its tenfold higher specific capacity than “graphite”, its low material density (0.534 g cm −3 ), and the most negative electrode potential (−3.04 V versus S.H.E. at 25 °C) [10,11]. Furthermore, the adoption of metallic Li as an anode is indispensable to realize high-energy-density batteries such as Li-O 2 and Li-S cells, both of which are being considered the “future of energy storage” [14,15,16,17,18,19,20,21,22,23,24,25]. Due to these promising prospects, a number of research projects focusing on the Li anode have been extensively carried out. Until now, only a few Li anode systems have shown promising results in terms of electrochemical stability and performance. For example, all-solid-state Li metal batteries with a thin Ag-C layer were successfully demonstrated by Samsung’s research group [26]. The designed pouch cell ran over 1000 cycles with maintaining a high energy density ( >900 Wh l −1 ) and an excellent Coulombic efficiency (99.8%). This approach presents a bright outlook where Li-metal systems can be practically adopted as next-generation energy storage. However, there are at least four representative challenges to overcome in order to make all solid-state LMBs commercially available in the market. (1) It is technically unable to form a perfect contact between the solid-state electrolyte and the electrode when fabricating solid-state batteries, which significantly increases the impedance at the electrode/electrolyte interface. (2) Not all the solid electrolytes have a good compatibility with electrodes, thus requiring an additional process to alter the surface nature of solid electrolytes. (3) Relatively slow kinetics of Li-ion transport through the solid-state electrolyte (in comparison with a liquid electrolyte) inhibits the practical use of Li metal anodes in the applications (e.g., EV) requiring fast charging and high power density [27,28]. (4) The manufacturability and material cost of solid-state batteries are not cost-efficient so far, in comparison with that of an liquid electrolyte system [29,30]. In this regard, it would be reasonable to take into account other systems using organic liquid electrolytes and understand what would happen if Li metal is employed in liquid electrolyte-based LIBs.
Metallic Li with “host-less” nature can be infinitely changing during Li plating/stripping process. This leads to a breakdown/restoration of an unstable and non-homogenous solid electrolyte interphase (SEI) layer over the surface of Li metal electrode [11,31,32,33,34]. During Li plating (or termed ‘deposition’), Li + flux is significantly intensified through the cracks of the SEI layer, giving rise to the formation of sharp Li dendrites [31,35,36,37]. The physical features of the formed Li dendrites could lead to the puncture of a polyolefin separator, which dramatically increases the risk of a battery short-circuit. Apart from the formation of Li dendrites, electrochemically dead and isolated Li pillars detached from the Li metal anode are floating inside the cell, which reduces the actual mass of electrochemically active Li ( Figure 1 a) [11]. All of these unwanted phenomena become even more serious when LMBs are operating under abuse conditions, i.e., high/low temperature, high current density, and overcharging. Especially, these topics are of importance since they can directly affect the safety of our lives [38,39,40,41].
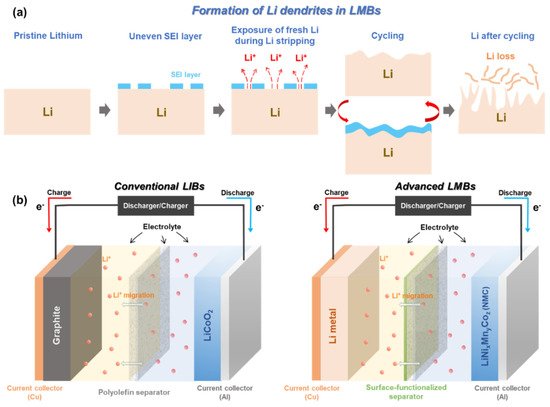
A number of strategies have been proposed to make LMB technology feasible and safer, such as stabilizing the Li electrode surface via interface modification [42,43,44,45,46,47], modifying the chemical constituents of electrolytes [48,49,50,51,52,53,54,55,56,57,58], adding a flame-retardant-based separator [59,60,61,62], and functionalizing the surface of polymer separators [23,63,64,65,66,67]. Encapsulating the Li metal with a protective layer through vapor deposition methods (e.g., physical vapor deposition) has shown a stabilized surface reactivity against atmospheric environments (air and humidity) and has presented optimistic electrochemical results in terms of cycle performance and Coulombic efficiency [43,44,45,46]. Nevertheless, the approach to modify the surface of Li metal via sophisticated techniques is technically hard to achieve both reproducibility and scalability. As to the electrolyte modification, it has somewhat shown impressive effects on suppressing the growth of Li dendrites, enhancing the Coulombic efficiency, and establishing the robust SEI layer [49,50,51]. Moreover, this approach is highly compatible with the conventional battery manufacturing process. Due to these benefits, it is considered the most feasible strategy to resolve the intrinsic problems of Li metal anodes. However, it itself cannot be a fundamental solution to prevent the battery short-circuit caused by Li dendritic growth. In addition, unavoidable side reactions including a parasitic reaction between a Li anode and liquid electrolyte seriously deteriorate the Coulombic efficiency and capacity retention of LMBs [68].
2. Conventional Polymer Separators for LIBs
The porosity, pore size, and thickness are crucial features determining the properties of separators and the overall performances of cells. In general, the pore size and porosity of typical separators is around 1 µm and 40%, respectively [73]. In order to use the separator for practical LMBs, the pore size is required to be small enough (e.g., sub-micrometer) to physically block the penetration of growing Li dendrites, and the porosity should be comparable to typical separators in order to facilitate efficient Li + transfer across the separator. In case of separator thickness, commercially available Celgard’s polymer separators have a thickness of around 25 µm [73]. If the total thickness of the separator gets thicker, it increases the overall resistance of the cell and reduces the loading amount of active materials in the cell. On the contrary, if the total thickness of the separator becomes thinner, it increases the risk of separator puncture by growing Li dendrites.
The battery separator should get wet as soon as it contacts liquid electrolytes. It is reported that the electrolyte wettability of conventional separators is highly associated with battery cycle retention and capacity [56,73,74]. If the separator has a poor electrolyte wettability, it would cause a non-uniform Li-ion transport across the separator. This would result in uneven Li deposition over the electrode, consequently leading to a short circuit of LMBs [75]. To facilitate the homogeneous Li deposition/dissolution in LMBs, it is of paramount importance to improve the electrolyte wettability of separators. The wetting behavior between a separator and a liquid electrolyte is typically studied by using the contact angle measurement [76]. If the separator has a good affinity with liquid electrolytes, the angle between the separator surface and the curvature of an electrolyte droplet would be small. On the other hand, if the separator has a bad affinity with liquid electrolytes, it would have a large contact angle. With these measurements, the electrolyte wetting behavior of separators can be directly determined.
For battery assembly, separators need to meet the following requirements: (1) high mechanical strength to endure the tension and pressure during the battery assembly, (2) excellent insulating property in order not to pass electrons across the separator, and (3) decent electrochemical/chemical stability to use the separator continuously for more than 1000 cycles without degradation [73,80,81]. Especially in the LMB system, the separator should be mechanically strong enough in the liquid cell to suppress the piercing of Li dendrites and the expansion of high-capacity electrodes such as Si and Li and to protect the entire cell from the external stress caused by physical shock and pressure. To evaluate these mechanical properties of separators, mechanical abuse tests (such as nail penetration, puncture, flat crush, and edge crush) and interface-bonding analyses are widely implemented across the industries [82,83,84,85,86,87].
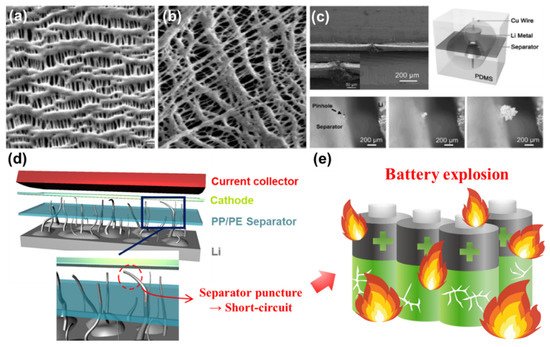
Polypropylene (PP) and polyethylene (PE) are two of the representative polyolefins typically used for fabricating conventional separators [74,80]. The separator fabricated by either wet process or dry process has a number of pores throughout the entire area ( Figure 2 a,b) [73]. Each has good mechanical strength and chemical stability against polar solvents. However, these separators are vulnerable to high temperature and have a relatively poor electrolyte wettability highly associated with the overall resistance in the cell, limiting the practical use for applications (such as EVs) requiring high current levels [73,81]. One of the other serious challenges when a Li metal anode is employed in rechargeable batteries is the separator puncture caused by the penetration of growing Li dendrites. This phenomenon was visually revealed by the transparent cell tests shown in Figure 2 c [67,88,89]. Note that extremely high current flows through the path where Li dendrites penetrate the separator, thus resulting in an exothermic reaction inside the cell followed by an explosion (or catching fire) of LMBs ( Figure 2 d,e) [90]. Typically, the average pore size (e.g., around 1 µm) of polyolefin separators is too large to prevent Li dendrites from piercing the separator. It is reported that the separator with a pore size less than 5 nm has a substantial effect on resisting Li dendrite penetration and redistributing Li + uniformly across the separator [91,92,93]. In order to meet the technical requirements described above and address the potential issues of LMBs, it is of importance to find the optimal condition for fabricating surface-functionalized separators that could maximize the stability and performance of LMBs.
3. Approaches to Modify the Surface of Conventional Polymer Separators for LMBs
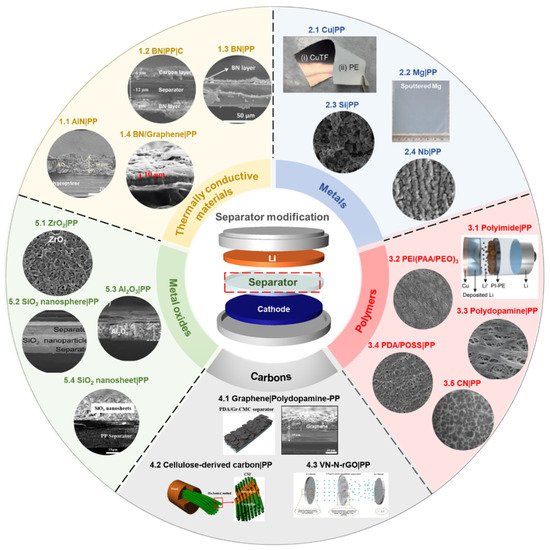
Among the important components of batteries, the battery separator has received relatively less attention than other parts in order to solve the systemic challenges of the Li anode system. The topic of modifying the surface of separators with functional materials has shown promising results in terms of cycle stability and Coulombic efficiency [63,67]. In addition, it is an efficient and straightforward strategy to control the morphology and growing orientation of Li dendrite. Typically, the coating materials to functionalize the surface of polymer separators can be classified into five or six types: (a) thermally conductive materials [63,64,69,94,95,96,97,98], (b) metals [99,100,101,102], (c) polymers [103,104,105,106,107,108], (d) carbons [66,109,110,111], (e) metal oxides [67,88,112,113,114,115,116,117,118,119], and (f) others [120,121,122,123,124] ( Figure 3 ). For electrochemical evaluation, each separator coating material is laminated onto one side or both sides of the polymer separator, using the tape-casting method, physical vapor deposition, etc. The information of separator-coating materials, separators, fabrication techniques, and electrochemical results is listed in the Table 1 .
However, the growth of dendritic Li is still unavoidable, even though the carbon layer on the separator can effectively suppress or change the feature of Li dendrites. In this regard, it would be eventually necessary to do broader research on either resolving the fundamental issue of Li dendrite growth itself or perfectly confining Li dendrites within the anodic side, using a hybrid composition of carbon and other functional materials.
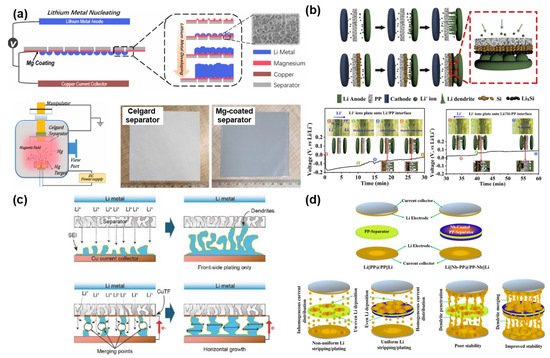
Unlike the previous research studies, there have been different attempts to use metals as functional layers for stabilizing Li anodes. Song et al. suggested a new strategy to control Li dendrite growth by forming a metal (e.g., Mg) layer on one side of the polyolefin separator via sputtering ( Figure 7 a) [101]. The Mg layer with a lithiophilic nature efficiently reduces the Gibbs free energy (ΔG) for Li plating, thus leading to a uniform and dense Li deposition over the counter electrode. The electrochemical results directly corroborated that the lithiophilic Mg layer is able to improve the polarization and cycle stability of the cells having a Li metal anode. Huang et al. demonstrated a “dendrite-eating” separator by coating a Si layer over the PP separator ( Figure 7 b) [102]. The main role of the Si layer is to stabilize Li deposition and reduce the loss of available Li during the repetitive electrochemical reaction. According to the electrochemical data, the symmetrical cell with a Si-coated separator has a smaller polarization and a better cycle stability than the symmetrical cell with a pristine PP separator. This directly indicates the influence of the Si layer on improving the electrochemical performances of LMBs. Transparent symmetric batteries with and without a Si-coated separator were tested to monitor the morphological change of growing Li dendrites as a function of Li plating time. The cell with a Si-coated separator survived longer than the cell with a PP separator. It is because the Si coating not only plays a role in restraining the dendrite formation by alloying with Li dendrites but also distributes Li + flux homogeneously across the separator. Zhang et al. proposed depositing an ultrathin Cu film on a PE separator for improving the electrochemical stability ( Figure 7 c) [99]. The ultrathin Cu film can change the growth orientation of Li dendrites from the vertical direction to the horizontal direction as well as diminish the local current density by exposing more surface area of the Li layer. Due to these beneficial functions of the ultrathin Cu layer, the cycle performance and Coulombic efficiency of Li|Cu cells were dramatically ameliorated in comparison with the Li|Cu cell with a pristine PE separator. Murugan et al. reported a binder-free Nb-coated PP separator for LMBs ( Figure 7 d) [100]. The Nb layer alters the surface nature of the PP separator from hydrophobicity to hydrophilicity, contributing to an improved electrolyte wettability. In addition, the formed metal layer not only enhances the mechanical strength of the conventional PP separator but also serves as an additional conductive path inducing the merge of Li dendrites from both sides of the anode and Nb layer. Moreover, the improved contact between the Nb layer and Li anode and the homogeneous current flow efficiently reduces the interfacial resistance between the anode and electrolyte. All these advantageous features greatly mitigate the risk of a short circuit and improve the cycle life with small polarization in LMBs.
Polymers are extensively used as binding materials, separators, and electrolytes in rechargeable batteries due to the high mechanical stability, the excellent chemical resistance, and the good adhesion properties [136]. In addition to these purposes, polymers are also adopted as coating materials for separator modification.
4. Conclusions and Outlooks
Typical polyolefin separators are not physically robust enough to restrain the propagation of growing Li/Na dendrites, which allows the unavoidable penetration of dendritic Li through the separator. In addition, polyolefin separators are not thermally stable to maintain its structural integrity at high temperatures and have relatively poor electrolyte wettability. These material limits hinder the practical use of polymer separators for high-performance batteries with Li metal anode. The surface modification of polymer separators with functional materials can play an important role in homogenizing Li + flux, preventing a physical penetration of alkaline metal dendrite, and strengthening a thermal/mechanical stability of separators. This paper reviews the characteristics and limits of existing battery separators and summarizes the overall separator-coating materials that are effective in controlling the growth of dendritic Li and improving the efficiency of reversible Li + transport. Surface-functionalized separators designed for LMBs can be classified into five or six categories according to the types of separator coating materials: (a) thermally conductive materials, (b) metal oxides, (c) carbons, (d) polymers, (e) metals, and (f) others (including solid-state electrolytes). Each type of material has different material properties, directly leading to different electrochemical results when applied to the surface of polymer separators for LMBs.
To date, there has been meaningful progress in fabricating functional separators for next-generation batteries including Li-S batteries and Li/Na metal batteries. With the adoption of the customized separators, the battery’s electrochemical/thermal stability has been remarkably improved in comparison to the existing battery technologies. These technologies are currently used by global battery industries (e.g., LG Chem., Samsung SDI, SK Innovation, etc.). Unfortunately, further improvements are still required to use the surface-modified separator over the long term and reduce the side reactions that mostly happen inside the Li metal cell. For example, it is no longer possible to homogenize Li + flux and block the Li dendrite penetration once the coating layer is fully covered by metallic Li during the electrochemical test. It is because the Li-deposited layer itself can serve as another Li anode inside the cell.
Considering the potential problems, there are a few parts where researchers and engineers can be devoted to upgrading the previous approaches. For instance, constructing a hybrid film comprising more than two types of separator-coating materials (described in Figure 3 ) on the polymer separator might be a feasible strategy to have multiple synergistic effects on improving the reversibility and stability of LMBs. The combination of a gel electrolyte with a surface-functionalized separator would be another approach to address the interface issues such as a non-uniform contact between a coating layer and an electrode. Alongside these suggested ideas, there are many niche markets where researchers can contribute to the further improvement of battery separators for practical LMBs. In addition to these conceptual approaches, the cost reduction of manufacturing functional separators is another important factor to be considered for successful commercialization. Since most of the previous studies were mainly focused on fabricating the surface-functionalized separator on a laboratory scale (using either tape-casting or sputtering), it would significantly increase the manufacturing cost of functional separators compatible with the battery assembly process.
In this regard, there are still many hurdles for researchers to go through. The topic of surface-functionalized separators needs more relevant and meaningful research to overcome the inherent weaknesses of polymer separators and open a new route to customize the properties of polymer separators.
This entry is adapted from the peer-reviewed paper 10.3390/nano11092275
