Melanoma is reported as the 19th most common cancer worldwide, with estimated age-standardized incidence rates of 2.8–3.1 per 100,000. Although the origin is most frequently cutaneous, mucosal melanoma has been described several times in literature, and despite its rarity (only 1% of all melanomas), increasing attention is being paid to this disease form. Within this subgroup, melanomas of the uropoetic apparatus are a rarity among rarities. Indeed, less than 50 cases of primary melanoma originating from the urinary bladder have been described, and even less originating from the kidney, renal pelvis and urethra.
- melanoma
- mucosal melanoma
- urology
1. Introduction
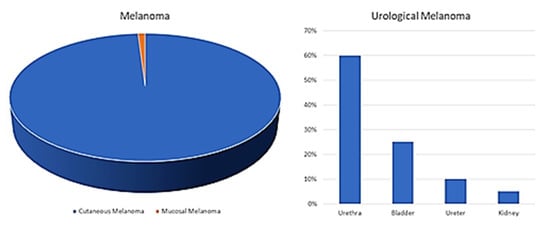
2. Discussion
2.1. Kidney
2.2. Ureter
2.3. Urinary Bladder
2.4. Urethra and Penis
3. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/cancers13174424
References
- World Health Organization (WHO). Classification of Skin Tumours; IARC: Lyon, France, 2018.
- Yde, S.S.; Sjoegren, P.; Heje, M.; Stolle, L.B. Mucosal Melanoma: A Literature Review. Curr. Oncol. Rep. 2018, 20, 28.
- Sánchez, R.B.; Bustos, B.D.U.; Mira, M.N.; Estrada, R.B. Mucosal Melanoma: An Update. Actas Dermo-Sifiliográficas 2015, 106, 96–103.
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 15, 1664–1678.
- Gru, A.A.; Becker, N.; Dehner, L.P.; Pfeifer, J.D. Mucosal melanoma: Correlation of clinicopathologic, prognostic, and molecular features. Melanoma Res. 2014, 24, 360–370.
- Nguyen, A.T.; Kavolius, J.P.; Russo, P.; Grimaldi, G.; Katz, J.; Brady, M.S. Primary genitourinary melanoma. Urology 2001, 57, 633–638.
- Acikalin, A.; Bagir, E.; Karim, S.; Bisgin, A.; Izol, V.; Erdogan, S. Primary melanoma of the urinary tract; Clinicopathologic and molecular review of a case series. Pathol. Res. Pract. 2020, 216, 153095.
- Fujimoto, H.; Chitose, K.; Tobisu, K.; Yamazaki, N.; Sakamoto, M.; Kakizoe, T. Solitary renal melanoma? A case with long survival after initial treatment. J. Urol. 1995, 153, 1887–1889.
- Frasier, B.L.; Wachs, B.H.; Watson, L.R.; Tomasulo, J.P. Malignant Melanoma of the Renal Pelvis Presenting as a Primary Tumor. J. Urol. 1988, 140, 812–814.
- Tajima, K.; Saito, K.; Umeda, Y.; Murata, T.; Satani, H. Malignant Melanoma of the Kidney Presenting as a Primary Tumor. Int. J. Urol. 1997, 4, 94–96.
- Hor Bayazit, Y.; Aridoğan, I.A.; Zeren, S.; Gönlüşen, G.; Tansug, Z. Primary malignant melanoma of the kidney. Scand. J. Urol. Nephrol. 2002, 36, 77–79.
- Tasdemir, C.; Samdanci, E.T.; Dogan, M.; Elmali, C.; Sargin, S.Y. Primer malignant melanoma of kidney: A case report. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 971–972.
- Ribalta, T.; Lloreta, J.; Munné, A.; Serrano, S.; Cardesa, A. Malignant pigmented clear cell epithelioid tumor of the kidney:Clear cell (“SUGAR”) tumor versus malignant melanoma. Hum. Pathol. 2000, 31, 516–519.
- Ainsworth, A.M.; Clark, W.H.; Mastrangelo, M.; Conger, K.B. Primary malignant melanoma of the urinary bladder. Cancer 1976, 37, 1928–1936.
- Stein, B.S.; Kendall, A.R. Malignant Melanoma of the Genitourinary Tract. J. Urol. 1984, 132, 859–868.
- Cunningham, J.A.; Fendler, J.-P.; Nichols, P.J.; Skinner, D.G. Metastatic malignant melanoma: An unusual case presentation. Urology 1994, 44, 924–926.
- Boughan, K.M.; Setrakian, S.; Lee, C.H.; Spiro, T.P.; Daw, H.A. A Renal Mass in a Patient with Melanoma. Clin. Genitourin. Cancer 2009, 7, E98–E100.
- Klatte, T.; Rao, J.Y.; Ribas, A.; Pantuck, A.J. Metastatic Melanoma to the Kidney Presenting with Renal Vein Tumor Thrombus. Urology 2007, 69, 982.e7–982.e9.
- Levin, B.M.; Boulos, F.I.; Herrell, S.D. Metastatic ocular melanoma to the kidney 20 years after initial diagnosis. Urology 2005, 66, 658.e11–658.e12.
- Makhlouf, H.R.; Ishak, K.G.; Shekar, R.; Sesterhenn, I.A.; Young, D.Y.; Fanburg-Smith, J.C. Melanoma markers in angiomyo-lipoma of the liver and kidney: A comparative study. Arch. Pathol. Lab. Med. 2002, 126, 49–55.
- Esheba Gel, S.; Esheba Nel, S. Angiomyolipoma of the kidney: Clinicopathological and immunohistochemical study. J. Egypt. Natl. Canc. Inst. 2013, 25, 125–134.
- Judd, R.L. Melanoma of the Ureter: A Case Report. J. Urol. 1962, 87, 805–807.
- Garcia, A.; Monserrat, J.M.; Martin, G.G. Melanoma of the ureter. Rev. Argent. Urol. Nefrol. 1969, 38, 58–61.
- Khan, M.; O’Kane, D.; Du Plessis, J.; Hoag, N.; Lawrentschuk, N. Primary malignant melanoma of the urinary bladder and ureter. Can. J. Urol. 2016, 23, 8171–8175.
- Gakis, G.; Merseburger, A.S.; Sotlar, K.; A Kuczyk, M.; Sievert, K.-D.; Stenzl, A. Metastasis of malignant melanoma in the ureter: Possible algorithms for a therapeutic approach. Int. J. Urol. 2009, 16, 407–409.
- MacNeil, J.; Hossack, T. A Case of Metastatic Melanoma in the Ureter. Case Rep. Urol. 2016, 2016, 1853015.
- March, B.; Calopedos, R.J.S.; Latif, E.; Ouyang, R. Ureteric Obstruction from Malignant Melanoma in Both Right Double Moiety and Left Single Moiety Ureters. Urology 2017, 103, e7–e8.
- Willis, A.J.; Huang, A.H.; Carroll, P. Primary Melanoma of the Bladder: A Case Report and Review. J. Urol. 1980, 123, 278–281.
- Anichkov, N.M.; Nikonov, A.A. Primary Malignant Melanomas of the Bladder. J. Urol. 1982, 128, 813–815.
- Goldschmidt, P.; Py, J.M.; Kostakopoulos, A.; Jacqmin, D.; Grosshans, E.; Bollack, C. Primary Malignant Melanomas of the Urinary Bladder. BJU Int. 1988, 61, 359.
- Van Ahlen, H.; Nicolas, V.; Lenz, W.; Boldth, I.; Bockisch, A.; Vahlensieck, W. Primary melanoma of urinary bladder. Urology 1992, 40, 550–554.
- El Ammari, J.E.; Ahallal, Y.; El Fassi, M.J.; Farih, M.H. Primary malignant melanoma of the urinary bladder. Case Rep. Urol. 2011, 2011, 932973.
- Schindler, K.; Schicher, N.; Kunstfeld, R.; Pehamberger, H.; Toepker, M.; Haitel, A.; Hoeller, C.; Harmankaya, K. A rare case of primary rhabdoid melanoma of the urinary bladder treated with ipilimumab, an anti-CTLA 4 monoclonal antibody. Melanoma Res. 2012, 22, 320–325.
- Lange-Welker, U.; Papadopoulos, I.; Wacker, H. Primary Malignant Melanoma of the Bladder. A case report and literature review. Urol. Int. 1993, 50, 226–228.
- Pacella, M.; Gallo, F.; Gastaldi, C.; Ambruosi, C.; Carmignani, G. Primary malignant melanoma of the bladder. Int. J. Urol. 2006, 13, 635–637.
- Truong, H.; Sundi, D.; Sopko, N.; Xing, D.; Lipson, E.J.; Bivalacqua, T.J. A Case Report of Primary Recurrent Malignant Mel-anoma of the Urinary Bladder. Urol. Case Rep. 2013, 12, 2–4.
- Karabulut, Y.Y.; Erdogan, S.; Sayar, H.; Ergen, A.; Baydar, D.E. Primary malignant melanoma of the urinary bladder: Clinical, morphological, and molecular analysis of five cases. Melanoma Res. 2016, 26, 616–624.
- Laudisio, A.; Giua, R.; Papalia, R.; Taffon, C.; Muto, G.; Incalzi, R.A. An Unusual Cause of Hematuria: Primary Bladder Melanoma in an Older Man. J. Am. Geriatr. Soc. 2016, 64, e122–e123.
- Buscarini, M.; Conforti, C.; Incalzi, R.A.; Falavolti, C.; Taffon, C.; Muto, G.; Dianzani, C. Primary Malignant Melanoma of the Bladder. Skinmed 2017, 15, 395–397.
- Singh, V.; Gupta, K.; Dewana, S.; Mandal, A.K. Spotting the pigmented ‘Monster’: Primary melanoma in urinary bladder. BMJ Case Rep. 2019, 23, e231950.
- Kirigin, M.; Lež, C.; Šarčević, B.; Šoipi, Š.; Jaić, G.; Ulamec, M.; Krušlin, B. Primary Malignant Melanoma of the Urinary Bladder: Case Report. Acta Clin. Croat. 2019, 58, 180–182.
- Snajdar, E.; Ajo, A.R.; Rosen, K.; Miller, R.; Mohammed, S.; Gordon, C.; Pui, J.C.; McIntosh, G. Primary Malignant Melanoma of the Urinary Bladder. Cureus 2021, 23, e14067.
- Maeda, T.; Uchida, Y.; Kouda, T.; Matsui, H.; Kawahara, Y.; Sato, T.; Nakajima, F. Metastatic malignant melanoma of the urinary bladder presenting with hematuria: A case report. Hinyokika Kiyo Acta Urol. Jpn. 2008, 54, 787–790.
- Nohara, T.; Sakai, A.; Fuse, H.; Imamura, Y. Metastatic Malignant Melanoma of the Urinary Bladder: A Case Report. Jpn. J. Urol. 2009, 100, 707–711.
- Siroy, A.E.; MacLennan, G.T. Primary Melanoma of the Bladder. J. Urol. 2011, 185, 1096–1097.
- Efesoy, O.; Çayan, S. Bladder metastasis of malignant melanoma: A case report and review of literature. Med. Oncol. 2010, 28, 667–669.
- Meunier, R.; Pareek, G.; Amin, A. Metastasis of Malignant Melanoma to Urinary Bladder: A Case Report and Review of the Literature. Case Rep. Pathol. 2015, 2015, 173870.
- Theocharides, C.; Chatzopoulos, K.; Papanikolaou, D.; Siokas, V.; Amplianitis, I.; Papanikolaou, A. Metastatic Melanoma to the Urinary Bladder of Ocular Origin Accompanied with Primary Cutaneous Melanoma: Diagnostic Challenge—A Report of a Case. Case Rep. Pathol. 2017, 2017, 4818537.
- Paterson, A.; Sut, M.; Kaul, A.; Altieri, V.; Mutch, F.; Patel, J.; Sharma, H. Metastatic malignant melanoma of the urinary bladder: Case report and literature review. Cent. Eur. J. Urol. 2012, 65, 232–234.
- Ikeda, A.; Miyagawa, T.; Kurobe, M.; Uchida, M.; Kojima, T.; Tsutsumi, M.; Ito, S.; Sugita, S.; Nishiyama, H. Case of metastatic malignant melanoma of the urinary bladder. Hinyokika Kiyo Acta Urol. Jpn. 2013, 59, 579–582.
- Topal, C.; Kır, G.; Daş, T.; Sarbay, B.; Tosun, M.I. Metastatic malignant melanoma of the urinary bladder: A case report and review of the literature. Indian J. Pathol. Microbiol. 2016, 59, 532–534.
- Patil, R.V.; Woldu, S.L.; Lucas, E.; Quinn, A.M.; Francis, F.; Margulis, V. Metastatic Melanoma to the Bladder: Case Report and Review of the Literature. Urol. Case Rep. 2017, 11, 33–36.
- Barillaro, F.; Camilli, M.; Dessanti, P.; Chiesa, F.G.N.; Villa, A.; Pastorino, A.; Aschele, C.; Conti, E. Primary melanoma of the bladder: Case report and review of the literature. Arch. Ital. Urol. Androl. 2018, 30, 224–226.
- Montes, F.G.; Gómez, M.F.L.; Boyd, J. Does primary melanoma of the bladder exist? Actas Urológicas Españolas 2000, 24, 433–436.
- Lee, C.S.D.; Komenaka, I.K.; Hurst-Wicker, K.S.; DeRaffele, G.; Mitcham, J.; Kaufman, H.L. Management of metastatic malignant melanoma of the bladder. Urology 2003, 62, 351.
- Chaus, F.M.; Craig, M.; Bracamonte, E.; Sundararajan, S.; Lee, B.R. Primary Malignant Melanoma of the Bladder Treated by Robotic Partial Cystectomy and Immunotherapy. J. Endourol. Case Rep. 2019, 5, 151–153.
- Rapisarda, S.; Bada, M.; Polara, A.; Crocetto, F.; Creta, M.; Chiancone, F.; Occhipinti, M.; Bertoloni, R.; Marciano, A.; Aresu, L.; et al. Conservative management of primary malignant melanoma of the bladder: A case report. J. Med. Case Rep. 2021, 15, 1–4.
- Konigsberg, H.A.; Gray, G.F. Benign melanosis and malignant melanoma of penis and male urethra. Urology 1976, 7, 323–326.
- Yamamoto, N.; Maeda, S.; Takeuchi, T.; Tokuyama, H.; Kanematsu, M.; Kuriyama, M.; Ban, Y.; Kawada, Y.; Mizoguchi, Y.; Kasahara, M. Malignant melanoma of male urethra: A case report. Hinyokika Kiyo Acta Urol. Jpn. 1989, 35, 121–126.
- Primus, G.; Soyer, H.P.; Smolle, J.; Mertl, G.; Pummer, K.; Kerl, H. Early ‘Invasive’ Malignant Melanoma of the Glans penis and the Male Urethra. Report of a case and review of the literature. Eur. Urol. 1990, 18, 156–159.
- Calcagno, L.; Casarico, A.; Bandelloni, R.; Gambini, C. Primary malignant melanoma of male urethra. Urology 1991, 37, 366–368.
- Fujimoto, N.; Oda, M.; Shimoe, S. Primary Malignant Melanoma of the Male Urethra. Urol. Int. 1991, 47, 176–177.
- Ander, H.; Esen, T.; Tellaloğlu, S.; Uysal, V. Successful management of malignant melanoma of male urethra with local excision and adjuvant radiochemotherapy. Prog. Clin. Biol. Res. 1991, 370, 379–383.
- Arai, K.; Joko, M.; Kagebayashi, Y.; Tsumatani, K.; Kimura, S.; Sasaki, K.; Samma, S.; Okajima, E.; Nakaoka, S. Primary ma-lignant melanoma of the female urethra: A case report. Jpn. J. Clin. Oncol. 1993, 23, 74–77.
- Kim, C.J.; Pak, K.; Hamaguchi, A.; Ishida, A.; Arai, Y.; Konishi, T.; Okada, Y.; Tomoyoshi, T. Primary malignant melanoma of the female urethra. Cancer 1993, 71, 448–451.
- Rashi, A.-M.; Williams, R.M.; Horton, L. Malignant melanoma of penis and male urethra Is it a difficult tumor to diagnose? Urology 1993, 41, 470–471.
- Aragona, F.; Maio, G.; Piazza, R.; Salmaso, R. Primary malignant melanoma of the female urethra: A case report. Int. Urol. Nephrol. 1995, 27, 107–111.
- Touyama, H.; Hatano, T.; Ogawa, Y. Primary malignant melanoma of the female urethra: A case report. Hinyokika Kiyo Acta Urol. Jpn. 1997, 43, 597–598.
- Girgin, C.; Tarhan, H.; Sezer, A.; Ermete, M.; Gürel, G. A large primary malignant melanoma of the female urethra. Urol. Int. 1999, 63, 198–200.
- Sánchez-Ortiz, R.; Huang, S.F.; Tamboli, P.; Prieto, V.G.; Hester, G.; Pettaway, C.A. Melanoma of the penis, scrotum and male urethra: A 40-year single institution experience. J. Urol. 2005, 173, 1958–1965.
- Katz, E.E.; Suzue, K.; Wille, M.A.; Krausz, T.; Rapp, D.E.; Sokoloff, M.H. Primary malignant melanoma of the urethra. Urology 2005, 65, 389.
- Kato, H.; Hayashi, K.; Saida, T.; Kontani, K.; Nishizawa, O. Urethral Advancement Procedure for Reconstruction after Excision of Male Parameatal Melanoma in situ. Urol. Int. 2005, 74, 183–184.
- Yoshizawa, T.; Kawata, N.; Sato, K.; Hirakata, H.; Igarashi, T.; Ichinose, T.; Yamaguchi, K.; Takahashi, S. Primary Malignant Melanoma of the Female Urethra. Urology 2007, 70, 1222.e13–1222.e16.
- Nakamoto, T.; Inoue, Y.; Ueki, T.; Niimi, N.; Iwasaki, Y. Primary amelanotic malignant melanoma of the female urethra. Int. J. Urol. 2007, 14, 153–155.
- Inoue, M.; Ishioka, J.-I.; Kageyama, Y.; Fukuda, H.; Higashi, Y. Primary malignant melanoma of the male urethra: A case report. Hinyokika Kiyo Acta Urol. Jpn. 2008, 54, 305–308.
- Comploj, E.; Palermo, S.; Trenti, E.; Lodde, M.; Mian, C.; Carella, R.; Pycha, A. Unexpected Long Survival in Primary Malignant Melanoma of the Male Urethra. Case Rep. Dermatol. 2009, 1, 93–99.
- Akbas, A.; Akman, T.; Erdem, M.R.; Antar, B.; Kilicaslan, I.; Şinasi, Y.Ö. Female Urethral Malignant Melanoma with Vesical Invasion: A Case Report. Kaohsiung J. Med. Sci. 2010, 26, 96–98.
- Yoshii, T.; Horiguchi, A.; Shirotake, S.; Tobe, M.; Tasaki, S.; Hayakawa, M.; Sumitomo, M.; Asano, T. A case of primary amelanotic malignant melanoma of the female urethra. Jpn. J. Urol. 2010, 101, 734–737.
- Cho, S.T.; Song, H.C.; Cho, B.; Choi, W.S.; Lee, W.K.; Lee, Y.S.; Lee, Y.G.; Kim, K.K.; Park, S.-H.; Kim, J.W. Primary Malignant Melanoma of the Female Urethra. Korean J. Urol. 2012, 53, 206–208.
- Karaman, H.; Yesil, Y. Primary melanoma of the male urethra. Turk. J. Urol. 2014, 39, 201–203.
- Maruyama, T.; Matsui, T.; Kobayashi, Y.; Kuwae, H. Case of primary malignant melanoma of the female urethra at age 94: A case report. Hinyokika Kiyo Acta Urol. Jpn. 2014, 60, 571–574.
- Papeš, D.; Altarac, S.; Arslani, N.; Rajković, Z.; Antabak, A.; Ćaćić, M. Melanoma of the glans penis and urethra. Urology 2014, 83, 6–11.
- Li, Y.; Yuan, H.; Wang, A.; Zhang, Z.; Wu, J.; Wei, Q. Malignant melanoma of the penis and urethra: One case report. World J. Surg. Oncol. 2014, 12, 340.
- Pandey, P.K.; Vijay, M.K.; Goel, H.; Shukla, S. Primary malignant melanoma of female urethra: A rare neoplasm. J. Cancer Res. Ther. 2014, 10, 758–760.
- Broussard, A.P.; Chaudoir, C.; Gomelsky, A. Urethral melanoma in an elderly woman. Int. Urogynecol. J. 2014, 26, 149–150.
- Safadi, A.; Schwalb, S.; Ben-Shachar, I.; Katz, R. Primary malignant urethral melanoma resembling a urethral caruncle. Urol. Case Rep. 2017, 15, 28–29.
- Suzuki, H.; Nakanishi, Y.; Yoshino, K.; Kataoka, M.; Fukushima, H.; Tobisu, K.; Koga, F. Primary malignant melanoma of the female urethra: A case report. Jpn. J. Urol. 2018, 109, 111–115.
- Davuluri, M.; Long, B.; Semple, S.; Villanueva-Siles, E.; Aboumohamed, A. Primary Urethral Melanoma: A Case Report and Literature Review. Urology 2018, 126, 1–4.
- Aoki, Y.; Soma, T.; Nakamura, Y.; Fukui, N.; Sakai, Y.; Kageyama, Y. Malignant melanoma of the male urethra with increased 5-S-cysteinyldopa: A case report. IJU Case Rep. 2019, 2, 215–217.
- Tokita, T.; Kawahara, T.; Ito, Y.; Tsutsumi, S.; Abe, K.; Namura, K.; Sano, F.; Shioi, K.; Takamoto, D.; Yumura, Y.; et al. Primary amelanotic malignant melanoma of the male urethra with inguinal lymph node metastasis successfully controlled by nivolumab: A case report. Urol. Case Rep. 2018, 18, 54–56.
- Maruyama, Y.; Sadahira, T.; Mitsui, Y.; Wada, K.; Tanimoto, R.; Kobayashi, Y.; Araki, M.; Watanabe, M.; Watanabe, T.; Nasu, Y. Red nodular melanoma of the penile foreskin: A case report and literature review. Mol. Clin. Oncol. 2018, 9, 449–452.
- Bansal, N.; Garg, G.; Vashist, S. Primary malignant melanoma of urethra mimicking as urethral caruncle. BMJ Case Rep. 2018, 2018, bcr2018226056.
- El-Safadi, S.; Estel, R.; Mayser, P.; Muenstedt, K. Primary malignant melanoma of the urethra: A systematic analysis of the current literature. Arch. Gynecol. Obstet. 2013, 289, 935–943.
- McComiskey, M.; Iavazzo, C.; Datta, M.; Slade, R.; Winter-Roach, B.; Lambe, G.; Sangar, V.K.; Smith, M. Balloon Cell Urethral Melanoma: Differential Diagnosis and Management. Case Rep. Obstet. Gynecol. 2015, 2015, 919584.
- Fahmy, O.; Scharpf, M.; Fend, F.; Stenzl, A.; Gakis, G. Feasibility of Penis-Preserving Surgery for Urethral Melanoma: Proposal for a Therapeutic Algorithm. Clin. Genitourin. Cancer 2015, 13, e411–e413.
- Sandru, F.; Draghici, C.; Predescu, T.; Constantin, M.M.; Petca, R.-C.; Constantin, T.; Petca, A.; Dumitrașcu, M.C. Regressive melanoma in a female patient: A case report. Exp. Ther. Med. 2020, 20, 87–90.
- Nakra, T.; Dadhwal, R.; Nayyar, R.; Rastogi, S.; Kakkar, A.; Sharma, M.C.; Yadav, R. Primary urethral small cell melanoma with neuroendocrine differentiation: A case report. J. Egypt. Natl. Cancer Inst. 2020, 32, 40.
- Burity, C.R.T.; Linica, S.B.; Saade, R.D.; Ferreira, F.T.; Schultz, L.; Bezerra, E.S.; Franco, H.C.; Oliveira, R.A.; Costa, M.V.S. Lo-calized primary melanoma of male urethra with a 4-year follow up. Urol. Case Rep. 2021, 10, 101702.
- Smith, H.G.; Bagwan, I.; Board, R.E.; Capper, S.; Coupland, S.E.; Glen, J.; Lalondrelle, S.; Mayberry, A.; Muneer, A.; Nugent, K.; et al. Ano-uro-genital mucosal melanoma UK national guidelines. Eur. J. Cancer 2020, 135, 22–30.
 Encyclopedia
Encyclopedia
