Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nutrition & Dietetics
Adipose tissue is a key organ in obesity etiology and the main storage site for carotenoids. We thus first describe carotenoid metabolism in adipocyte and adipose tissue and the effects of carotenoids on biological processes in adipose tissue that may be linked to obesity management in in vitro and preclinical studies.
- adipocytes
- adipose tissue
- brain
- carotenoids
- obesity
1. Obesity, Comorbidities, Adipose Tissue and Brain Dysfunctions
The World Health Organization (WHO) defines obesity and being overweight as abnormal or excessive fat accumulation that presents a risk to health [1]. The risk is mainly related to comorbidities strongly linked to obesity such as metabolic inflammation, insulin resistance, liver steatosis, hypertension, dyslipidemia, certain types of cancer, depression, etc. The WHO states that in 2016, around 39% of the adult population were overweight, and about 13% of the world’s adult population were obese [1]. This prefigures a major public health issue in the short term not only in western countries but also in low- and middle-income countries, where an epidemic of obesity and being overweight is emerging.
The excess fat mass that characterizes obesity is produced by an expansion of adipose tissue mediated by hypertrophy and/or hyperplasia of adipocytes [2], which is linked to complex, tightly regulated adipogenesis. This process has been studied in depth, and both the temporal sequences and the transcriptional regulators involved have been identified. Among them, the nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ) and the CCAAT-enhancer-binding protein (CEBP) families are considered as transcriptional regulators of adipogenesis [3]. Through this mechanism, the adipose tissue can participate in energy homeostasis, allowing the storage of excess energy as triglycerides (lipogenesis) and the release of energy as fatty acids (lipolysis). This balance is tightly regulated, and dysregulation may result in body weight gain or loss.
Several strategies have been proposed to fight obesity, including pharmacological approaches, limitation of fat and sugar consumption, promotion of physical activity and consumption of fruits and vegetables. Plant-based food is classically associated with weight management not only due to its macronutrient composition, but also to the presence of micronutrients, such as carotenoids. These substances correspond to a large family of C40 lipophilic pigments produced by plants, fungi and bacteria [20]. Carotenoids can be divided into two groups according to their chemical structure: carotenes, which are hydrocarbons, and xanthophylls, which also contain oxygen and are therefore less apolar than carotenes (Figure 1). More than 600 different substances have been identified, of which 50 can be found in the human diet, and of which only about 10 are present in significant amounts in human plasma [21]. Carotenoids containing an unsubstituted β-ionone ring are termed provitamin A, as they can be cleaved by animals to release retinal, which can subsequently be converted to retinol [20].
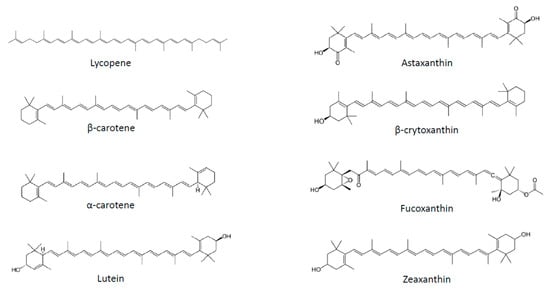
Figure 1. Chemical structures of the main carotenoids.
2. Carotenoids and Obesity in Human Studies
2.1. Observational Studies
Obesity has been associated in many epidemiological and observational studies with low circulating concentrations of carotenoids [22,23]. Strong inverse correlations between body mass index (BMI) and all measured carotenoids in plasma, except lycopene, were highlighted in the CARDIA study [24]. In addition, many obesity-associated disorders, such as low-grade inflammation or insulin resistance, are also strongly inversely associated with serum carotenoid concentrations [25,26,27].
2.2. Intervention Studies
Several trials have been conducted to study how carotenoids might be used in obesity management. Most of these studies used mixtures of carotenoids and vitamins in a natural matrix, such as fruit juices or plant extracts (reviewed by Bonet et al. [28]), making interpretation of the specific contribution of carotenoids difficult. To our knowledge, only two randomized double-blind placebo-controlled clinical trials have investigated the effect of pure carotenoid or xanthophyll supplementation. Canas et al. [29] reported a decrease in BMI z-score, waist-to-height ratio and subcutaneous adipose tissue in children given a mixture of carotenoids (β-carotene, α-carotene, lutein, zeaxanthin, lycopene, astaxanthin and γ-tocopherol) for 6 months. These beneficial effects were strongly associated with an increase in plasma β-carotene concentration in children [29]. Another study used a mixture of paprika xanthophylls and carotenoids, administered for 12 weeks to healthy overweight volunteers. This supplementation reduced visceral fat area, subcutaneous fat area and total fat area, along with BMI in the treated group compared to a placebo group [30].
3. Carotenoids and/or Metabolites are Involved in Body Weight Management and Limitation of Obesity Comorbidities in Preclinical Studies
Significant research has been devoted to studying the impact of β-carotene on energy metabolism and its outcome on obesity [31]. Its anti-obesity effect was subsequently demonstrated to be linked to a provitamin A effect [32,33], since β-carotene 15, 15′-monooxygenase (BCO) null mice did not display adipose tissue weight modification. This effect was found to be linked to decreased expression of PPARγ in adipose tissue and the involvement of retinoid X receptor (RAR) signaling in this regulation [34].
Astaxanthin prevented obesity in mice fed a high fat diet [35], via the limitation of adipose tissue expansion. Similar anti-obesity effects have been documented in mice fed a high fat and high fructose diet [36], where insulin sensitivity and inflammation were also improved by astaxanthin. Preventive effects of astaxanthin were found for hepatic steatosis [37] and inflammation and fibrosis in the liver in a non alcoholic steato hepatitis NASH and diet induced obesity (DIO) mice model [38].
Anti-adiposity properties have also been reported for β-cryptoxanthin [39], but their mechanism is still unknown. In addition, β-cryptoxanthin reversed liver steatosis and insulin resistance in DIO mice; this effect may be related to the anti-inflammatory effect of this carotenoid in the liver [40].
The potential of fucoxanthin for weight management has been extensively studied and reviewed [41]. This carotenoid limited weight gain and hyperglycemia, and inhibited the expression of several pro-inflammatory cytokines in adipose tissue of KK-a(y) mice [42]. Similar effects have been described in DIO mice, possibly through modulation of lipogenesis, adiponectin production and inflammation in adipose tissue [43], but also via browning of white adipose tissue [41].
The anti-obesity effect of lycopene was demonstrated in mice fed a high fat diet, where adiposity
was reduced after supplementation [1]. We and others have confirmed this beneficial
e ect of lycopene and/or tomato powder rich in lycopene in a DIO mice model on adiposity, glucose
homeostasis, adipose tissue and liver inflammation and steatosis [2][3].
was reduced after supplementation [1]. We and others have confirmed this beneficial
e ect of lycopene and/or tomato powder rich in lycopene in a DIO mice model on adiposity, glucose
homeostasis, adipose tissue and liver inflammation and steatosis [2][3].
4. Carotenoids and Adipocyte/Adipose Tissue Metabolism
4.1. Carotenoids Are Stored in Adipocytes and Adipose Tissue
It has long been known that carotenoids are notably stored in adipose tissue [51,52,53,54,55]. Lycopene and β-carotene are the predominant carotenoids in human adipose tissue [53,56]. More precisely, Chung et al. identified lycopene as the most abundant carotenoid in adipose tissue (more than 1/2), followed by β-carotene (approx. 1/3 of total carotenoids), lutein + zeaxanthin, β-cryptoxanthin and α-carotene [54].
Total carotenoid concentration appears to be site-specific, with abdomen concentration higher than in the buttocks or thigh [54]. Adipose tissue concentrations of carotenoids are similar in men and women [54]. Interestingly, plasma levels of most carotenoids are inversely correlated to fat mass and to both general and central adiposity [54,57], suggesting that during obesity, carotenoids are sequestered in adipose tissue. Conversely, weight loss is associated with an increase in lutein and zeaxanthin serum concentration [58]. In the case of β-carotene, it is noteworthy that even if its adipose tissue concentration is lower in obese people, the total pool of β-carotene is similar in obese and non-obese when taking into account total fat mass [59].
4.2. Carotenoids Are Metabolized in Adipocytes and Adipose Tissue
BCO1, involved in centric cleavage of carotenoids and β-carotene 9′, 10′-dioxygenase (BCO2), involved in eccentric cleavage of carotenoids, are expressed in adipocytes [71], raising the possibility that carotenoid cleavage products, including retinal, derivatives and apocarotenoids, could be found in adipocytes [31,32,72]. In agreement, retinal [73] and free retinol have been identified in the adipocyte fraction of adipose tissue [74]. Several isomers of retinol, including all-trans, 9-cis and 13-cis isomers, were also quantified in white adipose tissue [74,75,76], together with several isomers of retinoic acid, except for 9-cis retinoic acid [75,77,78]. Adipocytes express BCO1 and BCO2, together with the enzymes necessary for vitamin A metabolism, suggesting that part of the effect of provitamin A carotenoids is mediated via vitamin A production. This topic will not be dealt with here; the reader is referred to the excellent review of Dr. Blaner [50].
Besides these retinoids, β-10′-apocarotenal has been identified in adipose tissue [32]. It is highly probable that other apocarotenoids are produced in adipose tissue, but their function in adipocyte biology needs further research.
4.3. Carotenoids Regulate Gene Expression in Adipocytes and Adipose Tissue
Several molecular mechanisms mediating the effects of carotenoids on gene expression have been described and may be related to the impact of carotenoids on adipocyte biology. In the case of provitamin A carotenoids, leading to retinoic acid synthesis, RARs and retinoid X receptors (RXRs), they constitute specific signaling targets. Two families of receptors mediate the effects of retinoids [79,80]. Three subtypes of each have been described (RARα RARβ, RARγ, RXRα, RXRβ and RXRγ). These receptors work as ligand-dependent transcriptional regulators by binding specific DNA sequences—retinoic acid response element (RARE) or retinoid X response element (RXRE)—found in the promoter region of retinoid target genes either as RAR-RXR or RXR-RXR dimers. RAR and RXR subtypes are found in every cell type. Furthermore, RXRs are dimerization partners for other nuclear receptors such as peroxisome proliferator activated receptors (PPARs), liver X receptor (LXR), farnesoid X receptor (FXR), pregnane X receptor (PXR), RARs, thyroid hormone receptor (TR) and vitamin D receptor (VDR), which are involved in the regulation of a huge number of genes. In addition, several other transcription factors and signaling pathways are modulated by retinoic acid [81], including PPARβ ([82]. Lycopene [83] and apo-10′-lycopenoic acid [84] are also able to activate RAR. Many carotenoids regulate gene expression via ubiquitous signaling pathways such as nuclear factor-kappa B (NF-κB) and mitogen activated proteins (MAP) kinases [85,86], or via transcription factors involved in detoxification such as aryl hydrocarbon receptor (AhR), nuclear factor erythroid-2-related factor 2 (NRF2) or PXR [87,88].
5. Carotenoids and/or Metabolites Impact Adipocyte Biology In Vitro Studies
The impact of some carotenoids has been documented in adipogenesis (Figure 2), which could help obesity management via a limitation of lipid accumulation in adipocytes. Most of the reported effects inhibited adipocyte differentiation [89] by interfering with nuclear receptors such as RAR, RXR or PPAR. β-Carotene inhibited adipogenesis through the production of β-apo-14′-carotenal and repression of PPARα, PPARγ and RXR activation [90], but also through the production of all-trans retinoic acid [34]. Similarly, β-cryptoxanthin suppressed adipogenesis via activation of RAR [91], and astaxanthin inhibited rosiglitazone-induced adipocyte differentiation by antagonizing transcriptional activity of PPARγ [92]. Zeaxanthin [44] and fucoxanthin [93,94] exhibited anti-adipogenic effects via a down-regulation of adipogenic transcription factors C/EBPα and PPARγ, which blunted lipid accumulation. Conversely, lycopene (unpublished personal data) and apo-10′-lycopenoic acid [84] showed no effect on adipogenesis. Besides these effects, there is evidence that some effects of provitamin A carotenoids are mediated through retinol and its metabolite production, which are known to regulate adipogenesis [50].
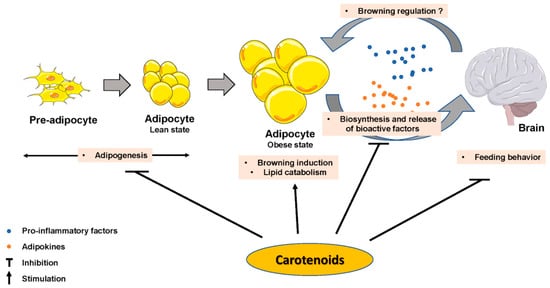
Figure 2. Carotenoids effect on adipose tissue biology parameters, on brain and on adipose tissue–brain crosstalk.
This entry is adapted from the peer-reviewed paper 10.3390/nu11071562
References
- Dhirendra Pratap Singh; P Khare; J Zhu; K K Kondepudi; J Singh; R K Baboota; Ravneet Boparai; R Khardori; K Chopra; M Bishnoi; et al. A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice. International Journal of Obesity 2015, 40, 487-496, 10.1038/ijo.2015.197.
- Soumia Fenni; Habib Hammou; Julien Astier; Lauriane Bonnet; Esma Karkeni; Charlène Couturier; Franck Tourniaire; Jean-François Landrier; Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders. Molecular Nutrition & Food Research 2017, 61, 1, 10.1002/mnfr.201601083.
- Cheng‐Chung Li; Chun Liu; Maobin Fu; Kang‐Quan Hu; Koichi Aizawa; Shingo Takahashi; Suganuma Hiroyuki; Junrui Cheng; Johannes Lintig; Xiang‐Dong Wang; et al. Tomato Powder Inhibits Hepatic Steatosis and Inflammation Potentially Through Restoring SIRT1 Activity and Adiponectin Function Independent of Carotenoid Cleavage Enzymes in Mice. Molecular Nutrition & Food Research 2018, 62, e1700738, 10.1002/mnfr.201700738.
This entry is offline, you can click here to edit this entry!
