Coordination polymers are solid-state structures consisting of repeating coordination units extending in one, two or three dimensions. Fields applications of the coordination polymers in general and metal-organic frameworks in particular are briefly discussed.
- coordination compounds
- metal-organic frameworks
- coordination bond
- ligands
1. Definition
Coordination polymers are solid-state structures consisting of repeating coordination units extending in one, two or three dimensions [1]. The first preparation and application of coordination polymers probably dates back to early 18th century, when German chemists accidentally discovered the Prussian blue dye [2]. Crystallographic studies, the first of which was carried out in 1936, revealed Prussian blue to be a 3D coordination polymer {[FeIII4FeII3(CN)18]·11.0H2O}n, in which the alternating Fe3+ and Fe2+ ions are linked by bridging cyanide ions [3].
Coordination polymers in which metal ions are linked by organic ligands into structures with potential voids are often referred to as metal-organic frameworks (MOFs) or porous coordination polymers [4][5]. The topology of coordination polymers can be tuned almost at will by careful choice of metal ions and organic linkers and a nearly infinite variety of structures can be obtained. Functional properties of the coordination polymers include capacity to store gases [6][7], separate gas [8][9][10] and liquid [11] mixtures, water purification [12][13], catalytic [14][15] and electrochemical [16][17][18] activities, biomedical applications [19][20][21][22].
2. Application
Luminescence is an important property of coordination polymers, often playing a key role in their applications. Luminescence is a non-coherent radiation that occurs upon the excitation of atoms, ions or molecules. Luminescence arises when certain transitions (called spontaneous radiative transitions) of these species from the states with higher energy to the states with lower energy, including the ground state, take place. Depending on the excitation method, different types of luminescence are differentiated. Thus, photoluminescence occurs upon excitation by an optical radiation (usually in UV range), electroluminescence—when excited by an electrical field. The processes that accompany the luminescence are often visualized in Jablonski diagrams (Figure 1). Absorption of light occurs in a very short femtosecond timeframe and correspond to the excitation of the particle from the ground state (S0) to an excited state (S1, S2, …). It should be noted that each state has its own set of vibrational levels, which are populated upon excitation with different probabilities and when combined, form an absorption spectrum. After the absorption of a photon, the most probable process is called the internal conversion or vibrational relaxation. This process is longer that the excitation (picosecond timeframe) and is accompanied by a structural relaxation of the excited molecule. The excess energy is converted into heat and the relaxation is thus a non-radiative process. The molecule can exist in this excited state for nanosecond and longer and then returns to the ground state, emitting a photon in a process called fluorescence. Other events that can occur after the excitation include non-radiative relaxation upon collision of the excited molecule with other particles or intersystem crossing to the lowest excited triplet state (T1). Relaxation from the triplet state to the ground state with photon emission is called phosphorescence. Transition back to the S1 state is also possible, followed by a delayed fluorescence.
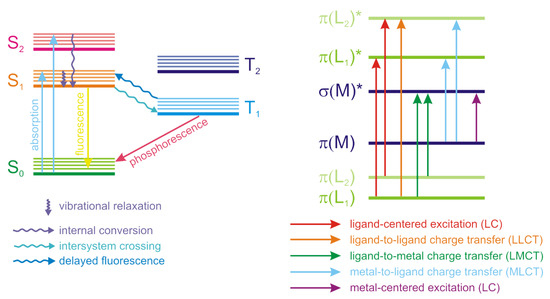
Figure 1. Jablonski diagram of processed accompanying absorption and emission of radiation (left) and schematic representation of energy levels in coordination polymers (right).
Coordination polymers are complex systems consisting of metal ions, one or more ligand types, inclusion of solvent molecules or other guests in voids is also possible. Emission of light by the coordination polymers can arise from various types of electron transitions—intraligand (ligand-centered), metal-centered, metal-to-ligand and ligand-to-metal charge transfer (MLCT and LMCT), Figure 1. Electron transitions in guest molecules encapsulated in the pores of the coordination polymers can also influence their photophysical properties.
3. Conclusion
The photophysical properties of the coordination polymers are used to create electroluminescent materials for LEDs [23][24], as contrast agents in biomedical imaging, theranostics and photodynamic therapy [25][26]. In recent years, more attention is given to nonlinear optical properties of the coordination polymers, including the second harmonic generation, multi-photon absorption, upconversion luminescence and lasing [27][28]. The most extensive area of the use of the luminescent properties of the coordination polymers is the development of sensors for various analytes - cations and anions in aqueous and non-aqueous solutions [29], gases (oxygen, nitric(II) oxide, carbon monoxide, ammonia, water vapor, etc.) [30], volatile organic compounds (aromatic hydrocarbons, aromatic nitro compounds, amines, etc.) [31], biologically important compounds (vitamins, pharmaceutical substances, toxins, DNA and RNA) [32]. The analytical signal in sensors of this type, as a rule, is associated either with a decrease in the luminescence intensity in the presence of an analyte (the “quenching” effect), or with its increase (the “turn-on” effect).
This entry is adapted from the peer-reviewed paper 10.3390/ma13122699
References
- Öhrström, Let’s talk about MOFs—Topology and terminology of metal-organic frameworks and why we need them. Crystals 2015, 5, 154–162.
- Kraft, On the Discovery and History of Prussian Blue. Bull. Hist. Chem. 2008, 32, 61–67.
- Grandjean, ; Samain, L.; Long, G.J. Characterization and utilization of Prussian blue and its pigments. Dalt. Trans. 2016, 45, 18018–18044.
- Butova, V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307.
- Furukawa, ; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444.
- Tsivadze, Y.; Aksyutin, O.E.; Ishkov, A.G.; Knyazeva, M.K.; Solovtsova, O.V.; Men’shchikov, I.E.; Fomkin, A.A.; Shkolin, A.V.; Khozina, E.V.; Grachev, V.A. Metal-organic framework structures: Adsorbents for natural gas storage. Russ. Chem. Rev. 2019, 88, 925–978.
- Lin, ; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296.
- Lin, -B.; Xiang, S.; Zhou, W.; Chen, B. Microporous Metal-Organic Framework Materials for Gas Separation. Chem 2020, 6, 337–363.
- Barnett, R.; Gonzalez, M.I.; Long, J.R. Recent Progress Towards Light Hydrocarbon Separations Using Metal–Organic Frameworks. Trends Chem. 2019, 1, 159–171.
- Iacomi, ; Formalik, F.; Marreiros, J.; Shang, J.; Rogacka, J.; Mohmeyer, A.; Behrens, P.; Ameloot, R.; Kuchta, B.; Llewellyn, P.L. Role of structural defects in the adsorption and separation of C3 hydrocarbons in Zr-fumarate-MOF (MOF-801). Chem. Mater. 2019, 31, 8413–8423.
- Pei, ; Shao, K.; Zhang, L.; Wen, H.-M.; Li, B.; Qian, G. Current Status of Microporous Metal–Organic Frameworks for Hydrocarbon Separations. Top. Curr. Chem. 2019, 377, 33.
- Li, ; Wang, H.; Yuan, X.; Zhang, J.; Chew, J.W. Metal-organic framework membranes for wastewater treatment and water regeneration. Coord. Chem. Rev. 2020, 404, 213116.
- Gao, ; Xu, J.; Bu, X.-H. Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2019, 378, 17–31.
- Kang, -S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.-Y. Metal–organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2019, 378, 262–280.
- Dhakshinamoorthy, ; Asiri, A.M.; García, H. Metal–Organic Frameworks as Multifunctional Solid Catalysts. Trends Chem. 2020, 2, 454–466.
- Wang, -G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093.
- Xie, -X.; Yang, Y.-C.; Dou, B.-H.; Li, Z.-F.; Li, G. Proton conductive carboxylate-based metal–organic frameworks. Coord. Chem. Rev. 2020, 403, 213100.
- Kempahanumakkagari, ; Vellingiri, K.; Deep, A.; Kwon, E.E.; Bolan, N.; Kim, K.-H. Metal–organic framework composites as electrocatalysts for electrochemical sensing applications. Coord. Chem. Rev. 2018, 357, 105–129.
- Cui, ; Ren, S.; Sun, B.; Jia, S. Optimization protocols and improved strategies for metal-organic frameworks for immobilizing enzymes: Current development and future challenges. Coord. Chem. Rev. 2018, 370, 22–41.
- Chowdhury, A. Metal-organic-frameworks for biomedical applications in drug delivery, and as MRI contrast agents. J. Biomed. Mater. Res. Part A 2017, 105, 1184–1194.
- Zhuang, ; Young, A.P.; Tsung, C.K. Integration of Biomolecules with Metal–Organic Frameworks. Small 2017, 13, 1–14.
- Banerjee, ; Lollar, C.T.; Xiao, Z.; Fang, Y.; Zhou, H.-C. Biomedical Integration of Metal–Organic Frameworks. Trends Chem. 2020, 2, 467–479.
- Zak, P.; Lapina, V.A.; Pavich, T.A.; Trofimov, A.V.; Trofimova, N.N.; Tsaplev, Y.B. Luminescent materials for modern light sources. Russ. Chem. Rev. 2017, 86, 831–844.
- Ahmad, ; Liu, J.; Ji, W.; Sun, L. Metal–Organic Framework Thin Film-Based Dye Sensitized Solar Cells with Enhanced Photocurrent. Materials 2018, 11, 1868.
- Pandey, ; Dhas, N.; Deshmukh, P.; Caro, C.; Patil, P.; Luisa García-Martín, M.; Padya, B.; Nikam, A.; Mehta, T.; Mutalik, S. Heterogeneous surface architectured metal-organic frameworks for cancer therapy, imaging, and biosensing: A state-of-the-art review. Coord. Chem. Rev. 2020, 409, 213212.
- Nguyen, N.; Ebrahim, F.M.; Stylianou, K.C. Photoluminescent, upconversion luminescent and nonlinear optical metal-organic frameworks: From fundamental photophysics to potential applications. Coord. Chem. Rev. 2018, 377, 259–306.
- Medishetty, ; Zaręba, J.K.; Mayer, D.; Samoć, M.; Fischer, R.A. Nonlinear optical properties, upconversion and lasing in metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 4976–5004.
- Razavi, A.A.; Morsali, A. Metal ion detection using luminescent-MOFs: Principles, strategies and roadmap. Coord. Chem. Rev. 2020, 415, 213299.
- Kumar, ; Kim, K.-H.; Rarotra, S.; Ge, L.; Lisak, G. The advanced sensing systems for NOx based on metal-organic frameworks: Applications and future opportunities. Trac. Trends Anal. Chem. 2020, 122, 115730.
- Rasheed, ; Nabeel, F. Luminescent metal-organic frameworks as potential sensory materials for various environmental toxic agents. Coord. Chem. Rev. 2019, 401, 213065.
- Liu, ; Xie, X.-Y.; Cheng, C.; Shao, Z.-S.; Wang, H.-S. Strategies to fabricate metal–organic framework (MOF)-based luminescent sensing platforms. J. Mater. Chem. C 2019, 7, 10743–10763.
- Wang, -L.; Xie, L.-H.; Joseph, E.A.; Li, J.-R.; Su, X.-O.; Zhou, H.-C. Metal–Organic Frameworks for Food Safety. Chem. Rev. 2019, 119, 10638–10690.
