Chitosan (CS) is a hemi-synthetic cationic linear polysaccharide produced by the deacetylation of chitin. CS is non-toxic, highly biocompatible, and biodegradable, and it has a low immunogenicity. Additionally, CS has inherent antibacterial properties and a mucoadhesive character and can disrupt epithelial tight junctions, thus acting as a permeability enhancer. As such, CS and its derivatives are well-suited for the challenging field of ocular drug delivery. In the present review article, we will discuss the properties of CS that contribute to its successful application in ocular delivery before reviewing the latest advances in the use of CS for the development of novel ophthalmic delivery systems. Colloidal nanocarriers (nanoparticles, micelles, liposomes) will be presented, followed by CS gels and lenses and ocular inserts. Finally, instances of CS coatings, aiming at conferring mucoadhesiveness to other matrixes, will be presented.
- chitosan
- derivatives
- ocular drug delivery
- ophthalmic applications
- mucoadhesion
- antibacterial
- nanoparticles
- hydrogels
- coatings
1. Introduction
Ocular diseases affect a growing number of people across the globe. Some pathological ophthalmic conditions, such as glaucoma, diabetic retinopathy, or age-related macular degeneration, cause severe visual impairment that can ultimately lead to blindness. In spite of their relative accessibility, or rather because of it, eyes are well-protected organs and successful ocular drug delivery is one of the important challenges the pharmaceutical industry has to face.
Ocular tissues are protected from foreign substances by a series of static and dynamic barriers and protective mechanisms, as illustrated in Figure 1 [1]. Tear turnover, reflex blinking, and nasolacrimal drainage drain agents away from the eye surface. The corneal epithelium and conjunctiva cover and protect the ocular surface. Additionally, the blood–ocular barriers (blood–aqueous and blood–retina) limit the access of compounds from systemic circulation. This defensive system is further assisted by enzymes and other barriers (corneal stroma, sclera, etc.).
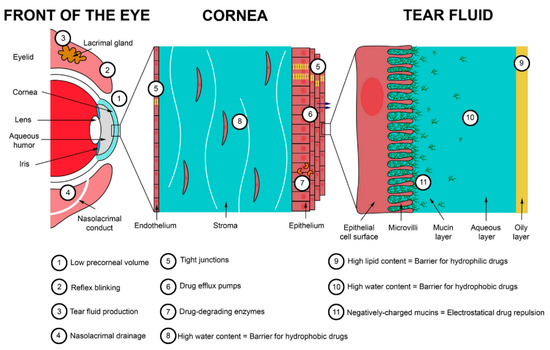
Figure 1. Main barriers to ocular delivery. Reprinted from ref. [2]. Copyright 2020 with permission from Elsevier.
Eye drops are the most frequently prescribed form of ocular treatment [3]. This is due to several inherent advantages of topical instillation of eye drops such as their non-invasive character and easy administration, resulting in high patient compliance and their immediate action. Despite their convenience, it is generally accepted that due to the ocular barriers, only 5% of the administered drug actually reaches its target, as shown in Figure 2 [4][5][6][7][8]. Moreover, only a small volume (approximately 30 μL) of eye drop formulation can be instilled in the eye. As a result, concentrated solutions have to be administered, and frequent instillations are necessary to reach a therapeutic result [2][9][10][11]. Systemic delivery is an alternative delivery method, yet not an ideal one as other limitations arise: first-pass metabolism, side effects, blood–ocular barriers, low eye vascularization, etc. In a quest toward more efficient drug delivery systems, the efforts have focused on increasing the ocular bioavailability of topical formulations. The generally accepted and less harmful solution to improve therapeutic treatment and drug bioavailability is to the increase the residence time of drop formulations on the ocular surface, thus increasing drug concentration on the cornea and reducing drug waste [12]. This has been achieved with drug-impregnated contact lenses, patches, colloidal carriers (nanoparticles, different kind of liposomes, etc.,), microneedles, films, gels, or solutions of mucoadhesive polymers, which is the simplest method [3][13][14][15][16][17][18][19][20][21][22][23]. A variety of materials has been used to prepare these smart delivery systems: natural or synthetic polymeric matrices, which can be modified and tuned for specific applications. Chitosan is one of them.
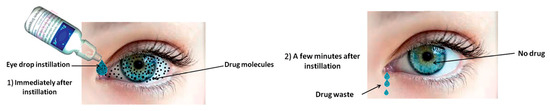
Figure 2. Drugs delivered through conventional eye drops. The main proportion of drug is typically removed after minutes whereas only a few percent absorbed into the eye. Reprinted from ref. [24]. Rights managed by Taylor & Francis.
Chitosan (CS) is a hemi-synthetic cationic linear polysaccharide made of randomly distributed D-glucosamine and N-acetyl-D-glucosamine units, linked via β-(1→4) glycosidic bonds. It is synthesized by the deacetylation of chitin, which is a naturally occurring polysaccharide. CS is water-soluble in acidic pH due to the protonation of the free amino groups. It is an easily accessible polymer, but most importantly it is non-toxic, highly biocompatible, and biodegradable, and it has a low immunogenicity [25]. As such, it is an excellent candidate for pharmaceutical and biomedical applications [26]. Besides, several CS-containing products have already been approved by the US Food and Drug Administration. CS has inherent antibacterial properties, a mucoadhesive character [27], and can disrupt epithelial tight junctions, thus acting as a permeability enhancer. Along its polymeric chains, CS bears free hydroxyl and amino groups, which can be used for its chemical modification, allowing for an easy tuning of its physicochemical properties. Finally, CS can easily be formulated in a wide range of forms such as micro and nanoparticles, films, membranes, sponges, gels, etc. [28][29][30][31][32]. All these characteristics render CS a particularly well-adapted polymer for ocular drug delivery.
2. Chitosan Properties
2.1. Mucoadhesion of CS and Its Derivatives
Several synthetic and natural polymers have mucoadhesive properties. These properties can be affected by numerous factors, such as the polymer’s chemical structure and the reactive groups concentration, which affect the hydrogen or ionic bonding ability, its molecular weight, chain flexibility, and its swelling and hydration ability. Mucoadhesive polymers have numerous reactive or charged groups (–COOH, –NH2, –OH, –SO3H, etc.) that are able to form non-covalent bonds with mucin and adhere to the mucosal surface. This process is called bioadhesion [33][34][35]. However, the mechanism of bioadhesion is not so simple and in order to understand it better, the mucus structure should be discussed beforehand.
Mucus is a weak viscoelastic gel that adheres to and covers the internal tracts of the body. Its main functions are to protect and lubricate epithelium damage and to impair microorganisms and other substances from passing into the body. From several studies, it was found that it consists of a mixture of water (about 95 wt %), 0.5–5 wt % glycoproteins (mucins), 0.5–1 wt % other proteins, about 1 wt % inorganic salts, and some small amounts of lipids and mucopolysaccharides [36]. Even though mucins are the main structural component of mucus, their detailed composition remains unclarified. However, it is estimated that the glycoproteins of mucin have a molecular weight ranging from 500 kDa to 20 MDa and are able to form a gel matrix due their association with each other via non-covalent hydrophobic interactions, hydrogen bonding between sugar units, and disulfide linkages between cysteine residues. This gel is responsible for the viscoelastic properties of mucus.
Mucus, due to the carbohydrate-bound ester sulfate residues and the carboxyl groups of sialic acids on the mucin proteoglycans, has a net negative charge. The glycosylated regions of mucins are hydrophilic, whereas the non-glycosylated protein domains are hydrophobic. The interactions between mucoadhesive polymers and the mucus surface involve first the wetting and adsorption of the two surfaces to create an intimate surface contact. This first step is promoted by the hydrophilic groups that the mucoadhesive polymers bear, creating non-covalent bonds such as hydrogen or ionic bonds with mucus charged groups. In a second step, polymeric macromolecules and mucus glycoproteins can interfuse or interpenetrate to a certain extent across the formed interface, thus strengthening the mucoadhesion contact.
Even though ionic and hydrogen bonding interactions (electronic interactions) are responsible for this adhesion, there are several other theories proposed to explain the mucoadhesion mechanism between a polymer and mucus, including: wetting, adsorption, diffusion interlocking, mechanical and fracture theory [37][38][39][40]. Most of them, as already discussed, accept that two phenomena such as surface energy thermodynamics and interpenetration or diffusion are responsible for enhanced mucoadhesion [41][42][43].
Numerous in vitro methods have been developed to evaluate the mucoadhesive properties of a polymer or pharmaceutical formulation [44][45]. Most methods are based on calculating the mucoadhesive strength by mechanical tests, and mainly by measuring the necessary force for the detachment of the formed interface between the mucus and polymer [40]. Incubation time is very important in order to allow enough time for hydrophilic and hydrophobic interactions to develop and achieve substantial interaction between a mucoadhesive polymer and a mucous surface. As reported in a recent study, after 10 min of incubation, the number of adhered CS nanoparticles (NPs) on a mucous surface is much higher, compared to a one-minute incubation or no incubation at all [46]. Furthermore, after washing, a longer incubation time results in an increased number of NPs remaining on the mucous surface.
Due to its cationic nature, CS has been extensively used as a mucoadhesive polymer [47][48][49]. The mechanism of molecular interactions between mucin and CS has recently been thoroughly described [50]. It was reported that mucoadhesion depends on external factors that include environmental conditions, such as the pH, the concentration, ionic strength, the ratio between macromolecules, the temperature and incubation time, as well as the properties of mucin (kind of mucus) and CS, such as surface charges, molecular weight, spatial conformation, flexibility of macromolecular chains, degree of deacetylation, ability of hydration and swelling, etc. [51]. CS and mucins interact predominantly electrostatically, yielding protein–polysaccharide complexes. However, the mucoadhesion of CS is pH-dependent. At very low pH, such as 1.2, CS shows poor mucoadhesion, since though its amino groups are completely protonated to –NH3+, the –COOH and –SO3H groups of the sialic acids of mucins are uncharged. At pH close to 4.5, the amino groups of CS are still protonated and thus electrostatic interactions such as ionic bonds take place with the negatively charged carboxylic and sulfate groups of mucin, which is the main mechanism of CS mucoadhesion [52]. At pH 7.0, all CS amino groups are deprotonated and their ability to electrostatically interact with the carboxylic and sulfonate groups of mucins is limited. Due to this behavior, it has been mentioned that mucoadhesive interactions are stronger at pH of 5.2 than 6.3; the calculated work forces of adhesion were 0.3–0.5 mN and 0.2 mN, respectively [53]. So, it is accepted that the protonated amino groups of CS are responsible for its mucoadhesivity, and when the number of these groups is reduced, as in the case of half-acetylated CS derivative, mucoadhesion is reduced, too [54][55]. In addition, electrostatic interactions cannot take place at neutral pH where the only existing interactions are hydrogen bonding and weak van der Waal’s forces.
The mucoadhesion strength of CS is 0.58 N/cm2, which is slightly lower than that of hydroxyethylcellulose (0.88 N/cm2) and much lower compared with poly(vinyl alcohol) (PVA) (5.11 N/cm2) [56]. However, it is high compared with poly(vinyl pyrrolidone) (PVP), which has a negligible mucoadhesive force, (6 mN/cm2), and hydroxypropyl methylcellulose (HPMC) (157 mN/cm2) [57]. In another study, the detachment force of neat CS from pig intestinal mucosa was found to depend on the kind of CS and ranged between 3.9 (low viscosity) and 6.7 mN/cm2 (high viscosity) [58]. In the same study, it was found that poly(acrylic acid) (PAA) had better mucoadhesive properties (11.7 mN/cm2), while other natural polymers such as pectin, starch and xanthan gum were not mucoadhesive. However, for PAA, much different mucoadhesive strength values have been reported such as 26 mN/cm2, which is comparable with that of poly(2-ethylhexyl acrylate) (21 mN/cm2) [59]. In the latter study, the effect of film contact time to mucus surface (10–300 s) and crosshead speed used for detachment (3–30 mm/min) were evaluated, and it was proved that the mucoadhesive strength increased by increasing both variables.
Taking into account that secondary bonding arises mainly from the existence of reactive groups, comparing CS and other mucoadhesive polymers, it was observed that those with –COOH groups such as carboxymethyl cellulose (CMC), alginic acid, and PAA had much better mucoadhesive properties than neat CS [51]. Frequently used mucoadhesive polymers have been classified according to their mucoadhesion strength: poly(acrylic acid)s (1–3.8 N/cm2) > alginate (1.1–2.8 N/cm2) > CS (0.42–0.85 N/cm2) > CMC (0.1–0.4 N/cm2) = HPMC (0.1–0.34 N/cm2) > gums (xanthan, badam, gellan, guar) (0.08–0.3 N/cm2) [60]. However, in another comparison of mucoadhesive properties of different polymers such as 2-hydroxyethyl ether cellulose, cellulose, HPMC, Kollidon VA 64, CS, carbopol 974 P NF, and Noveon AA-1, it was found that 2-hydroxyethyl ether cellulose and CS had the highest values of adhesion work, and that was attributed to the higher wettability of these polymers [61].
Besides its lower mucoadhesive strength, at neutral pH, CS has some additional limitations such as a low water solubility and low swelling properties. For example, the mucoadhesive strength of neat CS was estimated to 0.34 N/cm2 [62], while PAA (Carbopol 934) had a much higher mucoadhesive strength (about 0.51 N/cm2) [63]. To overcome these problems, CS has been modified with appropriate monomers, which have additional reactive groups to ensure mucoadhesion at pH 7 [64][65][66]. This is very important for ocular release formulations, since eye mucus has a pH about 7.8 and thus can be characterized as slightly basic surface, while nasal mucus has a neutral pH and in gastric mucus pH ranges from approximately 1–2 to 7 with pH rising from the luminal to the epithelial surface [67].
Trimethyl CS (TMCS) is maybe the most studied CS derivative. Due to its high cationic charge (-N+(CH3)3), it is one of the strongest mucoadhesive polymers [68]. The adhesion strength of TMCS is about 7–11 N/m2, and its mucoadhesion is due to interactions taking place between its positively charged quaternary ammonium groups and the negatively charged sulfate and sialic acid groups of mucosa [69]. An additional advantage of TMCS is its solubility in neutral and basic pH due to the alkylation of all amino groups with methyl groups, resulting in permanent positive charges. There are also amphoteric derivatives such as carboxymethyl CS, which due to the existence of both carboxyl and amino groups can act as an acidic or basic material, depending on the pH [70][71]. CS derivatives with additional reactive groups were also found to have enhanced mucoadhesive properties. For example, derivatives prepared with methylacrylate and acrylic acid (AA) exhibited improved mucoadhesion. This is due to the strong hydrogen bonds that the –COOH groups of AA can form with the –COOH and –SO3H groups of mucus glycoproteins; the improvement was much higher at pH 4 than at 6.4. For this reason, PAA is reported to have the highest mean adhesive force amongst other polymers [72].
It has been suggested that weak ionic or hydrogen bonding interactions between CS reactive groups and mucus groups are not able to provide sufficient mucoadhesion. For this reason, thiolated CS derivatives have been extensively studied [73][74][75][76][77][78][79][80]. These derivatives have free thiol groups that lead to the formation of covalent bonds with cysteine-rich subdomains of mucus glycoproteins [81]. Due to these stable bonds, thiolated CS derivatives possess excellent mucoadhesive properties and can increase the residence time of a polymer on a mucus surface. However, these polymers can only be applied in tissues that have a cysteine-rich mucus layer, since they need sulfhydryl groups to react. When N-hydroxysuccinimide was added on a polymer backbone, stable covalent bonds were formed with mucus amino groups, indicating that this could be an alternative method to prepare novel mucoadhesive excipients [82].
Similar interactions were also suggested between CS derivatives grafted with poly(ethylene glycol)diacrylate and a mucus surface [46][83]. These polyacrylate derivatives have free vinyl end groups, which may interact with the thiol groups of a mucin glycoprotein by a Michael-type reaction, forming covalent bonds. Due to these bonds, it was found that acrylated CS had better mucoadhesive properties than both CS and thiolated CS derivatives [84]. Other CS derivatives with catechol showed also enhanced mucoadhesive properties compared with CS due to the molecular complexation between the formed derivative surface and mucin [85]. Similar complexations have been reported in CS methacrylated derivatives, which were also found to have enhanced mucoadhesive properties [86].
Mucoadhesion seems to be a very promising approach to enhance drug effectiveness. However, there are some fundamental limitations to drug delivery through mucosal tissues [87]. Firstly, due to its high hydrophilicity and viscosity, mucus has multiple barrier properties and does not favor the diffusion of hydrophobic drugs [88]. Secondly, even though mucus gel is generally a stable system, it has a very short lifetime, since it is reformed dynamically through the secretion of mucins from goblet cells. It was found that the clearance period is about 5.0–7.7 min in the eye, 10–20 min in the respiratory tract, and much higher in the gastrointestinal tract: 4–6 h [39][89][90]. Thus, for several delivery systems, the mucoadhesive particles are not expected to adhere to mucus for long. Furthermore, despite the high mucoadhesive properties of some polymers, the capacity of mucus to immobilize foreign particles may become saturated. In other words, particles can only adhere on a mucus surface until the available surface area for adsorption is saturated [91]. It is currently accepted that while mucoadhesion is important to achieve drug bioavailability, the ability of polymeric structures to penetrate into mucus also plays an important role for drug effectiveness [92].
2.2. Antibacterial Properties
CS has antimicrobial activity since it can disrupt or destabilize the barrier properties of the outer membranes of Gram-negative bacteria or permeate the microbial plasma membrane [93][94][95][96]. The mechanism is based on interactions taking place between the positively charged amino groups of CS and the negatively charged microbial cell membranes [97]. These ionic interactions disrupt the microbial membrane, ultimately resulting in a leakage of intracellular constituents. The concentration of CS is very crucial, since when it is used at <0.2 mg/mL, the amino groups of CS interact with the negatively charged bacterial surface, leading to agglutination [98]. At higher concentrations, the number of CS amino groups is too high and can form a net positive charge onto the bacterial surfaces, resulting in a suspension.
CS derivatives have also antimicrobial properties against various microorganisms and, depending on the side groups, these properties can be substantially enhanced [97][99]. It is well known that the quaternization of CS can result in derivatives with enhanced antibacterial properties against several negative and Gram-positive bacteria compared to neat CS [100][101][102][103]. When comparing the inhibition of neat CS, TMCS, and N-diethylmethyl CS (DEMC) against Staphylococcus aureus, it was found that TMCS had the highest inhibition effect, followed by DEMC and CS [104]. This was attributed to the stronger positive charge of quaternary ammonium groups in TMCS compared with CS. These groups form strong polyelectrolyte complexes with negative peptidoglycans of the bacterial cell wall, leading to cell wall disruption and thus bacterial death. Quaternary CS derivatives prepared with 2-hydroxypropyltrimethyl ammonium chloride also showed high antimicrobial activity against S. aureus and Escherichia coli [105]. Furthermore, it was found that the antimicrobial activity increased by increasing the degree of quaternization. N,N,N-Trimethyl-O-(2-hydroxy-3-trimethylammonium propyl)-chitosans with different degrees of O-substitution were synthesized and demonstrated enhanced bacteriostatic properties against E. coli and S. aureus [106].
Except for quaternary CS derivatives, it was found that when carboxyl groups were grafted on CS chains, such as in the case of O-carboxymethyl CS (CMCS), antibacterial properties were further enhanced [99]. The introduction of sulfoxyamine groups in CS resulted in a derivative with simultaneously enhanced mucoadhesive and antibacterial properties [107].
2.3. Penetration Enhancement
Apart from antimicrobial and mucoadhesive properties, it was found that CS has also penetration-enhancing properties, since it can open the tight junctions located in the epithelial cells [108]. Cornea and conjunctiva have a negative charge; thus, the amino groups of CS could interact with these extraocular structures. This interaction could increase the drug concentration and its residence time, resulting in a higher accuracy of instilled drop solution and reproducibility of dosing [109][110]. According to numerous research works, CS-coated NPs can prolong the residence time in cornea and also enhance the intraocular penetration of drugs [111][112][113]. In a previous work, four quaternized CS derivatives (TMCS, dimethylethyl CS (DMEC), DEMC, and triethyl CS (TECS)) were prepared, and their properties as penetration enhancers evaluated [114]. It was found that transepithelial electrical resistance increased following the order: TMCS > DMEC > DEMC = TECS > CS, indicating their ability to open the tight junctions. In a comparative study, Mei et al. investigated the nasal absorption promoting effect of 2,3,5,6-tetramethylpyrazine phosphate using CS, TMCS, and thiolated CS of different molecular weights, for intranasal absorption [115]. It was found that TMCS was the strongest absorption enhancer, followed by neat CS, while thiolated CS could not improve the absorption properties of CS.
3. Chitosan Coatings
CS is a biocompatible polymer that, among others, can be used to enhance the mucoadhesion of drug formulations to tissues. Furthermore, the thiolation of CS can improve the penetration of NPs or their content through the tight junctions of mucus layers. Numerous studies have been conducted on the use of CS or its derivatives as coatings in order to deliver drugs encapsulated in liposomes, solid lipid matrixes, or NPs to the eye.
Li et al. encapsulated triamcinolone acetonide, which is an intermediate-acting glucocorticoid applied in inflammatory, edematous, and angiogenic ocular diseases, in liposomes, and the resulting NPs were further coated with CS [116]. The coating of NPs resulted in a slight increase of the size, i.e., from 108 to 135 nm, while the ζ-potential was inverted from −10 to +18 mV. Coated formulations were stable for a 60-day period at 4 °C, although a slight decrease (about 5%) in the drug entrapment efficiency was observed. Concerning the release profile of the drug, CS coating enhanced drug release compared to the uncoated NPs. Cellular uptake was enhanced by the CS coating, as evidenced by fluorescence microscopy (Figure 3). In vivo studies, performed on C57BL/6 mice, showed that the CS coating of liposomes led to a more efficient ocular delivery of triamcinolone acetonide to the posterior segment of the eye than eye drop formulation. Ocular toxicity tests showed that no toxicity was observed for either coated or uncoated liposomic NPs. The efficiency of these CS-coated liposomes in the treatment of retinal edema was further studied [117]. Experiments that were conducted in vivo using rat models concluded that CS-coated liposomes could deliver the drug into the retina of the eye. Furthermore, the formulation was found to effectively relieve retinal edema, caused by laser, without showing toxicity.
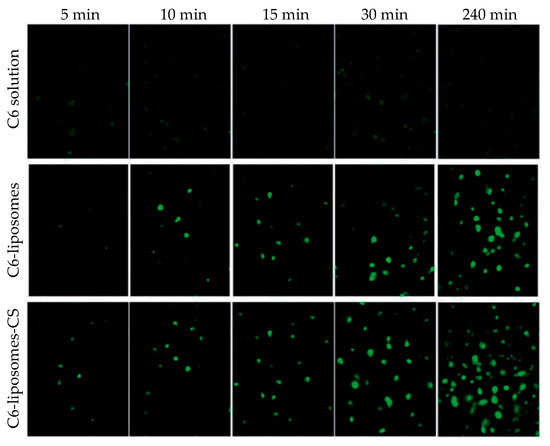
Figure 3. Cellular uptake of triamcinolone acetonide/coumarin 6 liposomes (C6-liposomes) and chitosan-coated triamcinolone acetonide/coumarin 6 liposomes (C6-liposomes-CS) by corneal epithelial cells at different times. Reprinted from ref. [116]. Rights managed by Taylor and Francis.
Tan et al. developed CS-coated liposomes for the ocular delivery of timolol maleate [118]. The drug-loaded liposomes were prepared using an ammonium sulfate gradient coupled with a pH gradient. The CS coating was applied simply by stirring the liposomes in a CS solution. Increasing the CS concentration afforded bigger particles with an initially increasing ζ-potential. Compared to timolol maleate eye drop formulation, in vitro release from the coated liposomes was extended over 12 h with a lower initial burst release. Transcorneal permeation was significantly enhanced, and ocular retention was improved as well. As a result, the IOP lowering efficiency was improved. Khalil et al. also prepared CS-coated liposomes for the nanoencapsulation of triamcinolone acetonide for posterior eye delivery [119]. The research team succeeded in preparing uncoated NPs of 18 nm in size while after coating with CS in concentrations of 0.1%, 0.2% and 0.3% w/v, their size increased to 100, 170, and 176 nm. In vivo studies were conducted in a rat model showing that after 15 days, the drug was present in the posterior chamber of the eye, which is a conclusion that is in accordance with the studies conducted by Li et al. [116] and Cheng et al. [117].
Chen et al. prepared CS-coated deformable liposomes for the ocular delivery of flurbiprofen [120]. The prepared NPs of deformable liposomes containing the drug were further coated by CS (Mw 50 kDa, degree of deacetylation: 95%) in three different concentrations (0.1%, 0.2%, and 0.4% w/v). It was found that the coated NPs were larger than the uncoated ones, and the ζ-potential changed from negative to positive. A corneal penetration study showed that deformable liposomes had a 1.5-fold increased penetration compared to non-deformable ones, while their penetration ability was further enhanced by the CS coating. Indeed, CS can improve the permeability of the cornea by opening the tight junctions among corneal epithelial cells or by intracellular routes (vide supra). A notable observation was that the penetration rate was different for the different formulations: all uncoated formulations showed a constant penetration rate, while the coated ones showed a reduced rate after 160 min. The cumulative penetration remained higher for the coated particles. In vivo pre-corneal retention showed that the CS coating significantly prolonged the residence time of deformable liposomes in the cornea and improved the drug bioavailability by increasing its transport time across the cornea.
Sun et al. studied for the first time the influence of the deacetylation degree of CS on corneal keratocyte adhesion, spreading, morphology, and integrin gene expression when used as a coating in pharmaceutical formulations [121]. The authors used CS with a molecular weight of 400 kDa, having three different degrees of deacetylation (74.1 ± 0.5%, 84.4 ± 0.7%, and 94.2 ± 0.5%). Initially, CS was 74% deacetylated. Further deacetylation was conducted via CS treatment with 60% (w/v) NaOH solution for 1 h once and for 1.5 h twice at 100 °C under a nitrogen atmosphere to obtain deacetylation degrees of 84% and 94%, respectively. FTIR and gel permeation chromatography showed that deacetylation did not affect the initial molecular weight. Crystallinity increased as the degree of deacetylation increased, which was due to the absence of acetyl groups. An important issue revealed by the study is that the degree of deacetylation affected cell adhesion, i.e., an increased deacetylation degree resulted in enhanced cell adhesion (Figure 4), which was probably due to the high stiffness and crystallinity of the biopolymers. It was suggested that the degree of deacetylation of CS coatings greatly affected cell adhesion-related phenomena and cell–substrate crosstalk during corneal keratocyte cultivation.
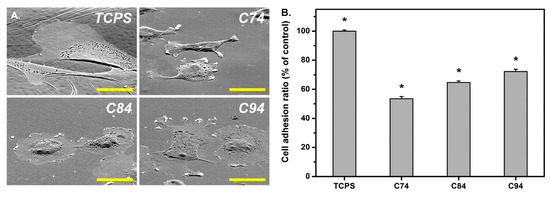
Figure 4. (A) Rabbit corneal keratocyte morphology on tissue culture polystyrene (TCPS) and chitosan substrates with various deacetylation degrees: 74% (C74), 84% (C84), and 94% (C94). Scale bar represents 30 μm. (B) Quantitative measurement of cell adhesion ratio for TCPS and various chitosan samples (* P b 0.05; n = 4). Data in the experimental groups are percentages relative to those of the TCPS groups. Reprinted from ref. [121]. Copyright 2016, with permission from Elsevier B.V.
Eid et al. studied the influence of pegylation and CS coating of ofloxacin lipid NPs [122]. Both the pegylation of lipid NPs and CS coating resulted in bigger NPs. Their shape was nearly spherical, with a smooth surface. Both pegylation and the CS coating resulted in a two- to threefold increase in the amount of ofloxacin that could be delivered to ocular fluids and tissues compared to commercial Oflox@ drops. Ban et al. reported the CS coating of dexamethasone-containing lipid NPs [123]. As a result of CS coating, the ζ-potential shifted from negative to positive values, and a higher permeation was observed. The lipid NPs exhibited a higher bioavailability compared to dexamethasone aqueous solution. Gelfuso et al. studied the use of iontophoresis as a method to increase voriconazole release, leading to an enhanced initial burst effect [124]. In brief, three different formulations were used: a cyclodextrin inclusion complex, a liposomal NP, and a CS-coated liposomal NP. After applying iontophoresis for 10 min, voriconazole penetration into the cornea was enhanced in all three formulations, with uncoated liposomal NPs showing the lowest concentration of the drug.
Seyfoddin et al. used a blend of different esters of behenic acid with glycerol (commercially available as Compritol® 888 ATO) as a lipid carrier for the nanoencapsulation of acyclovir, which is a drug that is used in the therapy of herpes keratitis: the most common infectious cause of blindness [125]. NPs were formed via the “hot microemulsion technique” and coated thereafter with CS, via their dispersion in solutions of different CS concentrations. The coating was achieved due to electrostatic interactions between the negatively charged lipid carrier and the positively charged CS. The authors studied the influence of the lyophilization process on the resulting NPs. It was observed that NPs tended to aggregate after freeze drying, resulting in higher sizes reaching the micro-scale range. Freshly prepared coated NPs showed small particle sizes between 323 and 468 nm; the size increased with increasing CS concentration. The ζ-potential was also proportional to the increase of CS concentration, starting from about −26 mV for uncoated NPs to about +28 mV when 1% w/v CS was used. No significant difference was observed between CS concentrations of 0.5% and 1% w/v. Drug release was studied in PBS pH 7.4. It was found that coating with CS led to a reduction of about 25% in acyclovir release rate compared to uncoated NPs, regardless of the CS concentration used for coating. The cellular uptake of NPs was enhanced by CS coating while the concentration of fluorescein increased with CS concentration and exposure time in the cytoplasm of the epithelial cells. In ex vivo penetration studies, conducted in bovine eyes, the coated NPs had enhanced properties, with the coating obtained from the 0.5% w/v CS concentration showing the best results. The quantification of acyclovir in cellular uptake was higher for the coated NPs.
Selvaraj et al. designed CS-coated NLCs for the ocular delivery of itraconazole [125]. The CS coating delayed itraconazole release due to the formation of a hydrophilic matrix around the lipid carriers, and ex vivo corneal permeability was significantly enhanced. In the antineovascularization study, CS-coated NLCs demonstrated a high reduction in neovascularization, which was attributed to low pre-corneal drainage, due to the higher mocoadhesivity and higher corneal permeation. Wang et al. used glyceryl monostearate as the solid lipid in the NP formulation of methazolamide used in glaucoma treatment [126]. Phospholipid was used as the surfactant in a modified oil-in-water emulsification technique, while CS was used for the coating of NPs. The results are in accordance with other studies; i.e., a smooth surface increased the particle size compared to uncoated NPs and prolonged drug release. In vivo results showed that coated NPs successfully delivered methazolamide in rabbit eyes, showing a marked decrease in IOP and a better sustainability than the uncoated ones.
Dukovski et al. investigated the performance of a cationic nanoemulsion containing ibuprofen for the treatment of dry eye disease, in order to resolve ibuprofen solubility complications and stabilize the tear film [127]. Nanoemulsions were prepared by microfluidization, using lecithin as an anionic surfactant, Miglyol 812 as an internal oil phase, Kolliphor EL as the second surfactant, and CS. CS was expected to depose on the surface of the nanoemulsion droplets, increasing the ζ-potential and thus mucoadhesion. The mucoadhesive properties of the obtained formulations were tested rheologically after mixing with mucin dispersion, and indeed, a significantly increased mucoadhesion was observed for the CS-coated nanoemulsion. The formulation further demonstrated good compatibility and stability. Nanoemulsions with 0.05% w/w CS exhibited the best characteristics and were found to be adequate for ophthalmic applications.
Another group that worked on liposomes coated with CS is Zhang et al. [128]. The group prepared liposomes via the reverse-phase evaporation method, which were subsequently coated with TMCS, 1 in Scheme 1. Cyanidin-3-glucoside was encapsulated in the liposomes for the treatment of cataract. The uncoated liposomes were negatively charged while a modified CS coating made their surface positively charged and simulteneously enlarged their size. Furthermore, the coating protected the drug from oxidative damage. The presence of TMCS increased the pre-corneal retention time, which was triplicated compared to uncoated liposomes. Moreover, it enhanced the permeation and the transepithelial transport of liposomes, as it opened the tight junctions between the epithelial cells. Regarding the release of the drug, a slower release and diffusion of the drug was observed owing to the presence of the coating. Huang et al. also used TMCS to coat lanosterol and hesperetin-loaded liposomes for cataract prevention and treatment [129]. The coated liposomes exhibited a slightly decreased encapsulation efficiency compared to the uncoated one. Most importantly, in in vivo studies, they demonstrated a smaller burst release and a slower overall release, extending over one week. Despite their lower drug load, the coated liposomes had a better therapeutic efficacy with no progression of cataract observed for the subjects treated with TMCS-coated liposomes.
Pai et al. used CS oligosaccharide in order to coat NLCs for the delivery of etoposide, which is an antineoplastic agent used for suppressing tumors that occur in the eye such as retinoblastoma [130]. NLCs were prepared via a hot homogenization–ultrasonication technique, and the resulting NPs were coated by CS oligosaccharides. Typical findings were also verified in the present study, i.e., an increase of NP size after CS coating (from 103 to 117 nm) and the conversion of negatively charged NPs to positively charged ones. However, compared to other analogous formulations, it was found that the release profile of the drug, conducted in STF, was not affected by CS oligosaccharide coating, which was probably due to the form of CS used, i.e., oligosaccharides. The mucoadhesion properties of coated NPs were enhanced compared to the negligible mucoadhesion of non-coated NPs. Mucoadhesion prolonged the presence of NPs on the ocular surface, as revealed by in vivo studies, leading to an enhanced ocular concentration of etoposide in all parts of the eye.
Li et al. studied three different CS derivatives—N-acetylcysteine CS (NACCS), 6 in Scheme 1, CS oligosaccharides, and carboxymethyl CS—for the coating of curcumin-loaded NLC [130]. According to curcumin release studies, NACCS showed a remarkably slower drug release compared to the other two coatings, which was probably due to the formation of disulfide bonds within the coating, impeding the diffusion of the drug. CS oligosaccharides showed a faster release compared to the thiolated CS coating; however, in contrast to Pai et al. [129], it was still slower than uncoated NLC. Finally, carboxymethyl CS showed the fastest release of all the coated NPs. Concerning corneal permeation studies, it was found that in the first 60 min, no difference was observed among the three coated formulations, but thereafter, thiolated CS, i.e., NACCS, showed an enhanced corneal permeation due to the thiol groups that can penetrate the tight junctions of mucus layers. These observations were further confirmed by fluorescence imaging, which was used to evaluate in vivo pre-corneal retention (Figure 5A). The same team further investigated the influence of the thiol group content in NACCS, which was used as a coating for curcumin-loaded NLC [131]. The total content of thiol groups did not affect curcumin’s release despite differences in the size and ζ-potential. This was attributed to the similar entrapment efficiency observed for all three coatings.
Differences appeared in ex vivo cornea penetration studies, which showed that after 60 min, the coating with the highest degree of thiolation resulted in higher cornea penetration. These outcomes were also confirmed by fluorescence imaging. A similar study was conducted for the ocular delivery of hydrophobic coumarin-6 instead of curcumin [132][133]. The results concerning both the coating with NACCS compared to CS oligosaccharides or carboxymethyl CS [131], as well as the content of thiol groups [133], were the same (Figure 5B).
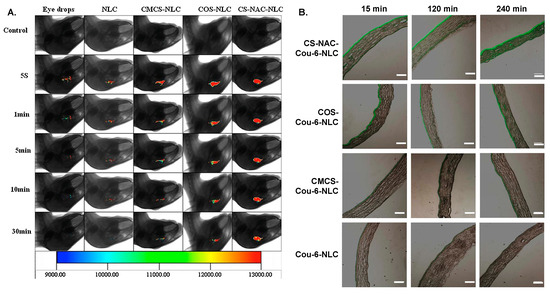
Figure 5. (A) In vivo fluorescence imaging of curcumin (CUR) in eye drops, nanostructured lipid carriers (NLC), carboxymethyl chitosan-coated NLC (CMCS-NLC), chitosan oligosaccharides-coated NLC (COS-NLC) and N-acetylcysteine chitosan-coated NLC (CS-NAC-NLC) at 5 s, 1 min, 5 min, 10 min, and 30 min after administration. Reprinted from ref. [130]. Copyright 2016 with permission from Elsevier Ltd. (B) Inverted fluorescence microscope micrographs after time-coursed in vivo corneal permeation of the aforementioned preparations, loaded with coumarin. Scale bar is 150 μm. Adapted from ref. [132]. Copyright 2017, with permission from Elsevier B.V.
Salama et al. used PLGA 50/50 or 75/25 in NP formulation of fluocinolone acetonide (FA), which is an anti-inflammatory corticosteroid drug used for the treatment of intermediate, posterior, and pan uveitis [134]. NPs were prepared by an innovative thin film hydration process using P407 in a mass ratio of PLGA/P407 1:5 and 1:10. The NPs prepared with PLGA 50/50 were smaller compared to the ones prepared with PLGA 75/25. This was attributed to the higher hydrophilicity of the former due to the higher glycolide content. This enabled the easier interfacial arrangement of PLGA molecules. Entrapment efficiency was higher for PLGA 75/25 NPs, which was due to the higher hydrophobic content of the polymer, which favored the encapsulation of the hydrophobic drug. The ζ-potential was negative for all prepared NPs due to the presence of terminal carboxylic acid groups in PLGA, and it increased with increasing poloxamer content. The authors selected the best formulation and proceeded in NPs coating using CS hydrochloride in different concentrations. It was observed, as it was by Seyfoddin et al. [125], that as the concentration of CS increased, the size of NPs increased from about 203 nm to about 2147 nm, while the ζ-potential also increased from negative to positive values. The important increase in size was attributed to agglomeration. Unfortunately, the isolation procedure of NPs was not described; it was probably by freeze drying, which could explain such sizes. The release profile revealed that the coated NPs showed improvements compared to the uncoated ones. In contrast to FA, which is known to cause irritation, an ocular irritation study conducted in rabbit eyes demonstrated that neither uncoated nor coated NPs induced any irritation. Furthermore, it was found that the concentration of FA in rabbits tears were higher when coated NPs were used. This was mainly attributed to the mucoadhesive properties of CS.
Pandit et al. studied CS-coated PLGA NPs for bevacizumab delivery [135]. Drug-loaded NPs were prepared by double emulsion solvent evaporation, experimental parameters were optimized by response surface methodology, and well-defined, spherical core–shell particles were obtained. CS coating contributed to a reduced initial burst release, a slower release compared to uncoated NPs, and a higher permeation. Khan et al. reported CS-coated PLGA NPs for the delivery of forskolin in glaucoma treatment [136]. CS-coated NPs were prepared in a single step by emulsion sonication. The experimental parameters were optimized using the Box–Behnken design. Increasing the CS concentration resulted in an increase in NPs’ size, a rise in PDI, and a decrease in drug loading, but an increase in entrapment efficiency. The optimized NPs were approximately 200 nm and had a positive ζ-potential. A slow release of forskolin was observed with no burst release. According to the authors, CS coating functioned as a physical barrier, restricting drug release. It is suggested that drug release was a result of CS swelling/erosion, followed by PLGA erosion and drug diffusion. Due to CS coating, the NPs were found to penetrate deeper in the cornea. Overall, CS-coated forskolin-loaded PLGA NPs showed a sustained IOP decrease for 24 h. Dyawanapelly et al. investigated the potential of CS oligosaccharide in the mucosal delivery of drug-loaded PLGA NPs [137]. In contrast to CS coating, CS oligosaccharide coating did not affect significantly drug release. Both coatings resulted in improved mucoadhesion and higher cellular uptake. Mahaling et al. studied the influence of different coatings on the bioavailability and distribution of NPs in the ocular region [138][139]. Initially, CS, gelatin, or pluronic F68 coatings of poly(ε-caprolactone) (PCL) NPs were studied, and the scope was then further extended to PLA and PLGA NPs. The NPs coated with F68 exhibited the highest hydrophilicity and mucoadhesivity. For PCL NPs, F68 was the best coating, but for PLGA NPs, CS-coated NPs demonstrated a higher bioavailability in conjunctiva, sclera, choroid, and retina. Nasr and Khoee reported the formation of a crosslinked CS shell, coating poly(butylene adipate) (PBA) micelles for the improved ocular delivery of loteprednol etabonate [140]. PBA was modified in order to prepare a dendrimerized structure with hydrophilic amine-functionalized chain ends that could form micelles. The amine-functionalized micelles were further reacted with N-succinyl modified CS, 2 in Scheme 1, in the presence of EDC and N-hydroxysuccinimide. CS was grafted to the micelles via the reaction of the amine groups of the micelles with the carboxylic acid groups of modified CS. Additionally, a crosslinking reaction among amine and carboxylic groups within CS chains took also place. Core–shell spherical NPs were obtained with sizes from 40 to 82 nm depending on the molecular weight of PBA. Commercial eye drops released their drug content within 10 h. In contrast, loteprednol etabonate release from the crosslinked core–shell NPs was extended over 50 h.
4. Concluding Remarks
CS is a versatile polymer due to its favorable biocompatibility, biodegradability, mucoadhesive, and penetration enhancement properties. Numerous applications and different drug delivery systems have been reported in the field of ocular drug delivery with promising results in terms of increased drug bioavailability and improved therapeutic effect. Not surprisingly, CS nanoparticles dominate amongst the different kind of delivery formulations. NPs have shown their superiority in drug delivery, generally speaking. In the challenging field of ocular delivery, the advantages of nanoparticles, such as their small size, high surface area, and high mucoadhesivity, are even more crucial. Ionotropic gelation, especially with TPP, is a convenient and easily accessible method to prepare CS nanoparticles and as such has attracted much attention. The importance of the experimental protocol, and more specifically the CS/TPP ratio and the CS and TPP concentrations, on the size, the dispersity, the ζ-potential (and thus stability), and the entrapment efficiency have been extensively discussed. Interesting performances have been obtained in terms of sustained release, and more are yet to come. In situ hydrogels are another type of formulation with encouraging results that have the advantage of being instilled as eye drops but that can form a hydrogel with a higher retention time once on the ocular surface. Innovative, more complex designs and architectures have started to emerge, combining complementary delivery vehicles; these need to be further investigated. These systems hold the promise of more efficient delivery as they combine multiple advantages. For example, NPs loaded in hydrogels or contact lenses can combine the controlled delivery of the NPs with a higher corneal retention time offered by the ocular lens or hydrogel, decreasing the necessity of frequent treatment. CS coating is another method to confer CS advantages to other matrices, without compromising their own advantages. The increase of therapeutic efficiency is the primary objective of any drug delivery system; nonetheless, patient’s comfort is almost as critical, since it heavily affects compliance to a treatment. This is especially true when it comes to ocular delivery. Thus, it should always be kept in sight when designing a drug delivery system.
This entry is adapted from the peer-reviewed paper 10.3390/polym12071519
References
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part I—Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75.
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22.
- Souto, E.B.; Dias-Ferreira, J.; López-Machado, A.; Ettcheto, M.; Cano, A.; Espuny, A.C.; Espina, M.; Garcia, M.L.; Sánchez-López, E. Advanced formulation approaches for ocular drug delivery: State-of-the-art and recent patents. Pharmaceutics 2019, 11, 460.
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Yañez, F.; Concheiro, A. Ocular drug delivery from molecularly-imprinted contact lenses. J. Drug Deliv. Sci. Technol. 2010, 20, 237–248. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, J.; Gonçalves, L.M.D. Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef]
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef]
- Duxfield, L.; Sultana, R.; Wang, R.; Englebretsen, V.; Deo, S.; Rupenthal, I.D.; Al-Kassas, R. Ocular delivery systems for topical application of anti-infective agents. Drug Dev. Ind. Pharm. 2016, 42, 1–11. [Google Scholar] [CrossRef]
- Khare, A.; Grover, K.; Pawar, P.; Singh, I. Mucoadhesive polymers for enhancing retention in ocular drug delivery: A critical review. Rev. Adhes. Adhes. 2014, 2, 467–502.
- White, C.J.; Tieppo, A.; Byrne, M.E. Controlled drug release from contact lenses: A comprehensive review from 1965-present. J. Drug Deliv. Sci. Technol. 2011, 21, 369–384. [Google Scholar] [CrossRef]
- Fulgêncio, G.; De, O.; Viana, F.A.B.; Ribeiro, R.R.; Yoshida, M.I.; Faraco, A.G.; Da Silva Cunha-Júnior, A. New mucoadhesive chitosan film for ophthalmic drug delivery of timolol maleate: In vivo evaluation. J. Ocul. Pharmacol. Ther. 2012, 28, 350–358. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular drug delivery barriers—Role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, Y.S.; Rupenthal, I.D. Overcoming ocular drug delivery barriers through the use of physical forces. Adv. Drug Deliv. Rev. 2018, 126, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular drug delivery: Present innovations and future challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Samy, K.E.; Bernards, D.A.; Desai, T.A. Recent advances in intraocular sustained—Release drug delivery devices. Drug Discov. Today 2019, 24, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Agban, Y.; Thakur, S.S.; Mugisho, O.O.; Rupenthal, I.D. Depot formulations to sustain periocular drug delivery to the posterior eye segment. Drug Discov. Today 2019, 24, 1458–1469. [Google Scholar] [CrossRef]
- Maharjan, P.; Cho, K.H.; Maharjan, A.; Shin, M.C.; Moon, C.; Min, K.A. Pharmaceutical challenges and perspectives in developing ophthalmic drug formulations. J. Pharm. Investig. 2019, 49, 215–228. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Das, P.J.; Adhikari, P.; Marbaniang, D.; Pal, P.; Ray, S.; Mazumder, B. Novel drug delivery systems for ocular therapy: With special reference to liposomal ocular delivery. Eur. J. Ophthalmol. 2019, 29, 113–126. [Google Scholar] [CrossRef]
- Suri, R.; Beg, S.; Kohli, K. Target strategies for drug delivery bypassing ocular barriers. J. Drug Deliv. Sci. Technol. 2020, 55, 101389. [Google Scholar] [CrossRef]
- Ntohogian, S.; Gavriliadou, V.; Christodoulou, E.; Nanaki, S.; Lykidou, S.; Naidis, P.; Mischopoulou, L.; Barmpalexis, P.; Nikolaidis, N.; Bikiaris, D.N. Chitosan nanoparticles with encapsulated natural and Uf-purified annatto and saffron for the preparation of UV protective cosmetic emulsions. Molecules 2018, 23, 2107. [Google Scholar] [CrossRef] [PubMed]
- Tashakori-Sabzevar, F.; Mohajeri, S.A. Development of ocular drug delivery systems using molecularly imprinted soft contact lenses. Drug Dev. Ind. Pharm. 2015, 41, 703–713.
- Kumar, A.; Vimal, A.; Kumar, A. Why chitosan? From properties to perspective of mucosal drug delivery. Int. J. Biol. Macromol. 2016, 91, 615–622
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661.
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53.
- Green, S.; Roldo, M.; Douroumis, D.; Bouropoulos, N.; Lamprou, D.; Fatouros, D.G. Chitosan derivatives alter release profiles of model compounds from calcium phosphate implants. Carbohydr. Res. 2009, 344, 901–907. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Mendes, A.C.; Moreno, J.S.; Hanif, M.; Douglas, T.E.L.; Chen, M.; Chronakis, I.S. Morphological, mechanical and mucoadhesive properties of electrospun chitosan/phospholipid hybrid nanofibers. Int. J. Mol. Sci. 2018, 19, 2266. [Google Scholar] [CrossRef]
- Hafezi, F.; Scoutaris, N.; Douroumis, D.; Boateng, J. 3D printed chitosan dressing crosslinked with genipin for potential healing of chronic wounds. Int. J. Pharm. 2019, 560, 406–415. [Google Scholar] [CrossRef]
- Michailidou, G.; Ainali, N.M.; Xanthopoulou, E.; Nanaki, S.; Kostoglou, M.; Koukaras, E.N.; Bikiaris, D.N. Effect of poly(vinyl alcohol) on nanoencapsulation of budesonide in chitosan nanoparticles via ionic gelation and its improved bioavailability. Polymers 2020, 12, 1101.
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in mucoadhesive drug-delivery systems: A brief note. Des. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef]
- Shaikh, R.; Raj Singh, T.; Garland, M.; Woolfson, A.; Donnelly, R. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570.
- Boegh, M.; Nielsen, H.M. Mucus as a barrier to drug delivery—Understanding and mimicking the barrier properties. Basic Clin. Pharmacol. Toxicol. 2015, 116, 179–186.
- Peppas, N.A.; Buri, P.A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release 1985, 2, 257–275. [Google Scholar] [CrossRef]
- Mikos, A.G.; Peppas, N.A. Scaling concepts and molecular theories of adhesion of synthetic polymers to glycoproteinic networks. In Bioadhesive Drug Delivery Systems; Lenaerts, V.M., Gurny, R., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 25–42. [Google Scholar]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Bassi Da Silva, J.; Ferreira, S.B. de S.; de Freitas, O.; Bruschi, M.L. A critical review about methodologies for the analysis of mucoadhesive properties of drug delivery systems. Drug Dev. Ind. Pharm. 2017, 43, 1053–1070.
- Grabovac, V.; Guggi, D.; Bernkop-Schnürch, A. Comparison of the mucoadhesive properties of various polymers. Adv. Drug Deliv. Rev. 2005, 57, 1713–1723. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Bagan, J.; Paderni, C.; Termine, N.; Campisi, G.; Russo, L.L.; Compilato, D.; Di Fede, O. Mucoadhesive polymers for oral transmucosal drug delivery: A review. Curr. Pharm. Des. 2012, 18, 5497–5514.
- Kellaway, I.W. In vitro test methods for the measurement of mucoadhesion. In Bioadhesion Possibilities and Future Trends (APV, Band 25); Gurny, R., Junginger, H.E., Eds.; Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, Germany, 1990; pp. 86–92. [Google Scholar]
- Peppas, N.A.; Sahlin, J.J. Hydrogels as mucoadhesive and bioadhesive materials: A review. Biomaterials 1996, 17, 1553–1561. [Google Scholar] [CrossRef]
- Eliyahu, S.; Aharon, A.; Bianco-Peled, H. Acrylated chitosan nanoparticles with enhanced mucoadhesion. Polymers 2018, 10, 106.
- Van Der Lubben, I.M.; Verhoef, J.C.; Van Aelst, A.C.; Borchard, G.; Junginger, H.E. Chitosan microparticles for oral vaccination: Preparation, characterization and preliminary in vivo uptake studies in murine Peyer’s patches. Biomaterials 2001, 22, 687–694. [Google Scholar] [CrossRef]
- Mahmood, A.; Lanthaler, M.; Laffleur, F.; Huck, C.W.; Bernkop-Schnürch, A. Thiolated chitosan micelles: Highly mucoadhesive drug carriers. Carbohydr. Polym. 2017, 167, 250–258. [Google Scholar] [CrossRef]
- Pontillo, A.R.N.; Detsi, A. Nanoparticles for ocular drug delivery: Modified and non-modified chitosan as a promising biocompatible carrier. Nanomedicine 2019, 14, 1889–1909.
- Collado-González, M.; González Espinosa, Y.; Goycoolea, F.M. Interaction between chitosan and mucin: Fundamentals and applications. Biomimetics 2019, 4, 32.
- Lohani, A.; Chaudhary, G. Mucoadhesive microspheres: A novel approach to increase gastroretention. Chronicles Young Sci. 2012, 3, 121.
- Barbu, E.; Verestiuc, L.; Nevell, T.G.; Tsibouklis, J. Polymeric materials for ophthalmic drug delivery: Trends and perspectives. J. Mater. Chem. 2006, 16, 3439–3443.
- Meng-Lund, E.; Muff-Westergaard, C.; Sander, C.; Madelung, P.; Jacobsen, J. A mechanistic based approach for enhancing buccal mucoadhesion of chitosan. Int. J. Pharm. 2014, 461, 280–285.
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Chitosan-based mucoadhesive tablets for oral delivery of ibuprofen. Int. J. Pharm. 2012, 436, 602–610.
- Nafee, N.A.; Boraie, N.A.; Ismail, F.A.; Mortada, L.M. Design and characterization of mucoadhesive buccal patches containing cetylpyridinium chloride. Acta Pharm. 2003, 53, 199–212.
- Karavas, E.; Georgarakis, E.; Bikiaris, D. Application of PVP/HPMC miscible blends with enhanced mucoadhesive properties for adjusting drug release in predictable pulsatile chronotherapeutics. Eur. J. Pharm. Biopharm. 2006, 64, 115–126.
- Lehr, C.M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48.
- Shojaei, A.H.; Paulson, J.; Honary, S. Evaluation of poly(acrylic acid-co-ethylhexyl acrylate) films for mucoadhesive transbuccal drug delivery: Factors affecting the force of mucoadhesion. J. Control. Release 2000, 67, 223–232.
- De Sá, L.L.F.; Nogueira, N.C.; Filho, E.C.D.S.; Figueiras, A.; Veiga, F.; Nunes, L.C.C.; Soares-Sobrinho, J.L. Design of buccal mucoadhesive tablets: Understanding and development. J. Appl. Pharm. Sci. 2018, 8, 150–163.
- Bartkowiak, A.; Rojewska, M.; Hyla, K.; Zembrzuska, J.; Prochaska, K. Surface and swelling properties of mucoadhesive blends and their ability to release fluconazole in a mucin environment. Colloids Surf. B 2018, 172, 586–593.
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev. Ind. Pharm. 2004, 30, 985–993.
- Abu-Huwaij, R.; Obaidat, R.M.; Sweidan, K.; Al-Hiari, Y. Formulation and in vitro evaluation of xanthan gum or carbopol 934-based mucoadhesive patches, loaded with nicotine. AAPS PharmSciTech 2011, 12, 21–27.
- Chopra, S.; Mahdi, S.; Kaur, J.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J. Advances and potential applications of chitosan derivatives as mucoadhesive biomaterials in modern drug delivery. J. Pharm. Pharmacol. 2006, 58, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, C.; Momin, M.; Gharat, S.; Omri, A. Functionalized and graft copolymers of chitosan and its pharmaceutical applications. Expert Opin. Drug Deliv. 2017, 14, 1189–1204. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267.
- Greaves, J.L.; Wilson, C.G. Treatment of diseases of the eye with mucoadhesive delivery systems. Adv. Drug Deliv. Rev. 1993, 11, 349–383.
- Muzzarelli, R.A.A.; Tanfani, F. The N-permethylation of chitosan and the preparation of N-trimethyl chitosan iodide. Carbohydr. Polym. 1985, 5, 297–307.
- Karavasili, C.; Katsamenis, O.L.; Bouropoulos, N.; Nazar, H.; Thurner, P.J.; van der Merwe, S.M.; Fatouros, D.G. Preparation and characterization of bioadhesive microparticles comprised of low degree of quaternization trimethylated chitosan for nasal administration: Effect of concentration and molecular weight. Langmuir 2014, 30, 12337–12344.
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tokura, S.; Tamura, H.; Selvamurugan, N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Prog. Mater. Sci. 2010, 55, 675–709. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. The implications of recent advances in carboxymethyl chitosan based targeted drug delivery and tissue engineering applications. J. Control. Release 2014, 186, 54–87.
- Aggarwal, S.; Agarwal, S. Mucoadhesive polymeric platform for drug delivery: A comprehensive review. Curr. Drug Deliv. 2015, 12, 139–156
- Hunt, G.; Kearney, P.; Kellaway, I.W. Mucoadhesive polymers in drug delivery systems. In Drug Delivery Systems: Fundamentals and Techniques; Johnson, P., Lloyd Jones, J., Ellis, H., Eds.; Ellis Horwood: Chichester, UK, 1987; pp. 180–199. [Google Scholar]
- Bernkop-Schnürch, A.; Steininger, S. Synthesis and characterisation of mucoadhesive thiolated polymers. Int. J. Pharm. 2000, 194, 239–247. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Scholler, S.; Biebel, R.G. Development of controlled drug release systems based on thiolated polymers. J. Control. Release 2000, 66, 39–48. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Hornof, M.; Guggi, D. Thiolated chitosans. Eur. J. Pharm. Biopharm. 2004, 57, 9–17. [Google Scholar] [CrossRef]
- Langoth, N.; Kahlbacher, H.; Schöffmann, G.; Schmerold, I.; Schuh, M.; Franz, S.; Kurka, P.; Bernkop-Schnürch, A. Thiolated chitosans: Design and in vivo evaluation of a mucoadhesive buccal peptide drug delivery system. Pharm. Res. 2006, 23, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Kongsong, M.; Songsurang, K.; Sangvanich, P.; Siralertmukul, K.; Muangsin, N. Design, synthesis, fabrication and in vitro evalution of mucoadhesive 5-amino-2-mercaptobenzimidazole chitosan as low water soluble drug carriers. Eur. J. Pharm. Biopharm. 2014, 88, 986–997. [Google Scholar] [CrossRef]
- Cho, I.S.; Oh, H.M.; Cho, M.O.; Jang, B.S.; Cho, J.-K.; Park, K.H.; Kang, S.-W.; Huh, K.M. Synthesis and characterization of thiolated hexanoyl glycol chitosan as a mucoadhesive thermogelling polymer. Biomater. Res. 2018, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nanaki, S.; Tseklima, M.; Christodoulou, E.; Triantafyllidis, K.; Kostoglou, M.; Bikiaris, D.N. Thiolated chitosan masked polymeric microspheres with incorporated mesocellular silica foam (MCF) for intranasal delivery of paliperidone. Polymers 2017, 9, 617.
- Bernkop-Schnürch, A. Thiomers: A new generation of mucoadhesive polymers. Adv. Drug Deliv. Rev. 2005, 57, 1569–1582.
- Menzel, C.; Hauser, M.; Frey, A.; Jelkmann, M.; Laffleur, F.; Götzfried, S.K.; Gust, R.; Bernkop-Schnürch, A. Covalently binding mucoadhesive polymers: N-hydroxysuccinimide grafted polyacrylates. Eur. J. Pharm. Biopharm. 2019, 139, 161–167.
- Eshel-Green, T.; Bianco-Peled, H. Mucoadhesive acrylated block copolymers micelles for the delivery of hydrophobic drugs. Colloids Surf. B 2016, 139, 42–51.
- Shitrit, Y.; Bianco-Peled, H. Acrylated chitosan for mucoadhesive drug delivery systems. Int. J. Pharm. 2017, 517, 247–255.
- Ryu, J.H.; Choi, J.S.; Park, E.; Eom, M.R.; Jo, S.; Lee, M.S.; Kwon, S.K.; Lee, H. Chitosan oral patches inspired by mussel adhesion. J. Control. Release 2020, 317, 57–66.
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Methacrylated chitosan as a polymer with enhanced mucoadhesive properties for transmucosal drug delivery. Int. J. Pharm. 2018, 550, 123–129.
- Bernkop-Schnürch, A. Mucoadhesive systems in oral drug delivery. Drug Discov. Today Technol. 2005, 2, 83–87.
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64.
- Lehr, C.M.; Poelma, F.G.J.; Junginger, H.E.; Tukker, J.J. An estimate of turnover time of intestinal mucus gel layer in the rat in situ loop. Int. J. Pharm. 1991, 70, 235–240. [Google Scholar] [CrossRef]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171.
- Ponchel, G.; Montisci, M.-J.; Dembri, A.; Durrer, C.; Duchêne, D. Mucoadhesion of colloidal particulate systems in the gastro-intestinal tract. Eur. J. Pharm. Biopharm. 1997, 44, 25–31.
- Zhang, X.; Cheng, H.; Dong, W.; Zhang, M.; Liu, Q.; Wang, X.; Guan, J.; Wu, H.; Mao, S. Design and intestinal mucus penetration mechanism of core-shell nanocomplex. J. Control. Release 2018, 272, 29–38.
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, P.; Kieft, T.L.; Ryan, S.J.; Baker, S.M.; Wiesmann, W.P.; Rogelj, S. Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomater. 2010, 6, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Michailidou, G.; Christodoulou, E.; Nanaki, S.; Barmpalexis, P.; Karavas, E.; Vergkizi-Nikolakaki, S.; Bikiaris, D.N. Super-hydrophilic and high strength polymeric foam dressings of modified chitosan blends for topical wound delivery of chloramphenicol. Carbohydr. Polym. 2019, 208, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63.
- Kandimalla, K.K.; Borden, E.; Omtri, R.S.; Boyapati, S.P.; Smith, M.; Lebby, K.; Mulpuru, M.; Gadde, M. Ability of chitosan gels to disrupt bacterial biofilms and their applications in the treatment of bacterial vaginosis. J. Pharm. Sci. 2013, 102, 2096–2101.
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419.
- Rúnarsson, Ö.V.; Holappa, J.; Nevalainen, T.; Hjálmarsdóttir, M.; Järvinen, T.; Loftsson, T.; Einarsson, J.M.; Jónsdóttir, S.; Valdimarsdóttir, M.; Másson, M. Antibacterial activity of methylated chitosan and chitooligomer derivatives: Synthesis and structure activity relationships. Eur. Polym. J. 2007, 43, 2660–2671. [Google Scholar] [CrossRef]
- Sadeghi, A.M.M.; Amini, M.; Avadi, M.R.; Siedi, F.; Rafiee-Tehrani, M.; Junginger, H.E. Synthesis, characterization, and antibacterial effects of trimethylated and triethylated 6-NH2-6-deoxy chitosan. J. Bioact. Compat. Polym. 2008, 23, 262–275. [Google Scholar] [CrossRef]
- De Britto, D.; Celi Goy, R.; Campana Filho, S.P.; Assis, O.B.G. Quaternary salts of chitosan: History, antimicrobial features, and prospects. Int. J. Carbohydr. Chem. 2011, 2011, 1–12. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Luan, F.; Yin, X.; Dong, F.; Li, Q.; Guo, Z. Synthesis of quaternary ammonium salts of chitosan bearing halogenated acetate for antifungal and antibacterial activities. Polymers 2018, 10, 530.
- Sadeghi, A.M.M.; Dorkoosh, F.A.; Avadi, M.R.; Saadat, P.; Rafiee-Tehrani, M.; Junginger, H.E. Preparation, characterization and antibacterial activities of chitosan, N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int. J. Pharm. 2008, 355, 299–306.
- Liu, P.; Meng, W.; Wang, S.; Sun, Y.; Aqeel Ashraf, M. Quaternary ammonium salt of chitosan: Preparation and antimicrobial property for paper. Open Med. 2015, 10, 473–478.
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S.; Liu, J. Synthesis, characterization, and antibacterial activity of N,O-quaternary ammonium chitosan. Carbohydr. Res. 2011, 346, 2445–2450.
- Jadhav, R.L.; Yadav, A.V.; Patil, M.V. Poly Sulfoxyamine grafted chitosan as bactericidal dressing for wound healing. Asian J. Chem. 2020, 32, 127–132.
- De la Fuente, M.; Raviña, M.; Paolicelli, P.; Sanchez, A.; Seijo, B.; Alonso, M.J. Chitosan-based nanostructures: A delivery platform for ocular therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 100–117.
- Eljarrat-Binstock, E.; Orucov, F.; Aldouby, Y.; Frucht-Pery, J.; Domb, A.J. Charged nanoparticles delivery to the eye using hydrogel iontophoresis. J. Control. Release 2008, 126, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Nagarwal, R.C.; Kant, S.; Singh, P.N.; Maiti, P.; Pandit, J.K. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J. Control. Release 2009, 136, 2–13.
- De Campos, A.M.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; El-Feky, G.S.; Kamel, R.; Awad, G.E.A. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for ocular drug delivery. Int. J. Pharm. 2011, 413, 229–236.
- Sadeghi, A.M.M.; Dorkoosh, F.A.; Avadi, M.R.; Weinhold, M.; Bayat, A.; Delie, F.; Gurny, R.; Larijani, B.; Rafiee-Tehrani, M.; Junginger, H.E. Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur. J. Pharm. Biopharm. 2008, 70, 270–278.
- Mei, D.; Mao, S.; Sun, W.; Wang, Y.; Kissel, T. Effect of chitosan structure properties and molecular weight on the intranasal absorption of tetramethylpyrazine phosphate in rats. Eur. J. Pharm. Biopharm. 2008, 70, 874–881.
- Li, J.; Cheng, T.; Tian, Q.; Cheng, Y.; Zhao, L.; Zhang, X.; Qu, Y. A more efficient ocular delivery system of triamcinolone acetonide as eye drop to the posterior segment of the eye. Drug Deliv. 2019, 26, 188–198.
- Cheng, T.; Li, J.; Cheng, Y.; Zhang, X.; Qu, Y. Triamcinolone acetonide-chitosan coated liposomes efficiently treated retinal edema as eye drops. Exp. Eye Res. 2019, 188, 107805.
- Tan, G.; Yu, S.; Pan, H.; Li, J.; Liu, D.; Yuan, K.; Yang, X.; Pan, W. Bioadhesive chitosan-loaded liposomes: A more efficient and higher permeable ocular delivery platform for timolol maleate. Int. J. Biol. Macromol. 2017, 94, 355–363.
- Khalil, M.; Hashmi, U.; Riaz, R.; Rukh Abbas, S. Chitosan coated liposomes (CCL) containing triamcinolone acetonide for sustained delivery: A potential topical treatment for posterior segment diseases. Int. J. Biol. Macromol. 2020, 143, 483–491.
- Chen, H.; Pan, H.; Li, P.; Wang, H.; Wang, X.; Pan, W.; Yuan, Y. The potential use of novel chitosan-coated deformable liposomes in an ocular drug delivery system. Colloids Surf. B 2016, 143, 455–462.
- Sun, C.C.; Chou, S.F.; Lai, J.Y.; Cho, C.H.; Lee, C.H. Dependence of corneal keratocyte adhesion, spreading, and integrin β1 expression on deacetylated chitosan coating. Mater. Sci. Eng. C 2016, 63, 222–230.
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Development, optimization, and in vitro/in vivo characterization of enhanced lipid nanoparticles for ocular delivery of ofloxacin: The influence of pegylation and chitosan coating. AAPS PharmSciTech 2019, 20, 1–14.
- Ban, J.; Zhang, Y.; Huang, X.; Deng, G.; Hou, D.; Chen, Y.; Lu, Z. Corneal permeation properties of a charged lipid nanoparticle carrier containing dexamethasone. Int. J. Nanomed. 2017, 2017, 1329–1339.
- Gelfuso, G.M.; Ferreira-Nunes, R.; Dalmolin, L.F.; Ana, A.C.; Dos Santos, G.A.; De Sá, F.A.P.; Cunha-Filho, M.; Alonso, A.; Neto, S.A.M.; Anjos, J.L.V.; et al. Iontophoresis enhances voriconazole antifungal potency and corneal penetration. Int. J. Pharm. 2020, 576, 118991.
- Seyfoddin, A.; Sherwin, T.; Patel, D.V.; McGhee, C.N.; Rupenthal, I.D.; Taylor, J.A.; Al-Kassas, R. Ex vivo and in vivo evaluation of chitosan coated nanostructured lipid carriers for ocular delivery of acyclovir. Curr. Drug Deliv. 2016, 13, 923–934.
- Wang, F.; Zhang, M.; Zhang, D.; Huang, Y.; Chen, L.; Jiang, S.; Shi, K.; Li, R. Preparation, optimization, and characterization of chitosan-coated solid lipid nanoparticles for ocular drug delivery. J. Biomed. Res. 2018, 32, 411–423.
- Jurišić Dukovski, B.; Juretić, M.; Bračko, D.; Randjelović, D.; Savić, S.; Crespo Moral, M.; Diebold, Y.; Filipović-Grčić, J.; Pepić, I.; Lovrić, J. Functional ibuprofen-loaded cationic nanoemulsion: Development and optimization for dry eye disease treatment. Int. J. Pharm. 2020, 576, 118979.
- Zhang, J.; Liang, X.; Li, X.; Guan, Z.; Liao, Z.; Luo, Y.; Luo, Y. Ocular delivery of cyanidin-3-glycoside in liposomes and its prevention of selenite-induced oxidative stress. Drug Dev. Ind. Pharm. 2016, 42, 546–553.
- Huang, C.; Li, C.; Muhemaitia, P. Impediment of selenite-induced cataract in rats by combinatorial drug laden liposomal preparation. Libyan J. Med. 2019, 14, 1548252.
- Pai, R.V.; Vavia, P.R. Chitosan oligosaccharide enhances binding of nanostructured lipid carriers to ocular mucins: Effect on ocular disposition. Int. J. Pharm. 2020, 577, 119095.
- Liu, D.; Li, J.; Pan, H.; He, F.; Liu, Z.; Wu, Q.; Bai, C.; Yu, S.; Yang, X. Potential advantages of a novel chitosan-N-acetylcysteine surface modified nanostructured lipid carrier on the performance of ophthalmic delivery of curcumin. Sci. Rep. 2016, 6, 1–14.
- Li, J.; Tan, G.; Cheng, B.; Liu, D.; Pan, W. Transport mechanism of chitosan-N-acetylcysteine, chitosan oligosaccharides or carboxymethyl chitosan decorated coumarin-6 loaded nanostructured lipid carriers across the rabbit ocular. Eur. J. Pharm. Biopharm. 2017, 120, 89–97. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Cheng, B.; Wu, Q.; Pan, H. Ex vivo and in vivo evaluation of the effect of coating a coumarin-6-labeled nanostructured lipid carrier with chitosan-n-acetylcysteine on rabbit ocular distribution. Mol. Pharm. 2017, 14, 2639–2648.
- Salama, A.H.; Mahmoud, A.A.; Kamel, R. A novel method for preparing surface-modified fluocinolone acetonide loaded plga nanoparticles for ocular use: In vitro and in vivo evaluations. AAPS PharmSciTech 2016, 17, 1159–1172.
- Pandit, J.; Sultana, Y.; Aqil, M. Chitosan-coated PLGA nanoparticles of bevacizumab as novel drug delivery to target retina: Optimization, characterization, and in vitro toxicity evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1397–1407.
- Khan, N.; Ameeduzzafar; Khanna, K.; Bhatnagar, A.; Ahmad, F.J.; Ali, A. Chitosan coated PLGA nanoparticles amplify the ocular hypotensive effect of forskolin: Statistical design, characterization and in vivo studies. Int. J. Biol. Macromol. 2018, 116, 648–663
- Dyawanapelly, S.; Koli, U.; Dharamdasani, V.; Jain, R.; Dandekar, P. Improved mucoadhesion and cell uptake of chitosan and chitosan oligosaccharide surface-modified polymer nanoparticles for mucosal delivery of proteins. Drug Deliv. Transl. Res. 2016, 6, 365–379.
- Mahaling, B.; Katti, D.S. Physicochemical properties of core-shell type nanoparticles govern their spatiotemporal biodistribution in the eye. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Mahaling, B.; Katti, D.S. Understanding the influence of surface properties of nanoparticles and penetration enhancers for improving bioavailability in eye tissues in vivo. Int. J. Pharm. 2016, 501, 1–9.
- Nasr, F.H.; Khoee, S. Design, characterization and in vitro evaluation of novel shell crosslinked poly(butylene adipate)-co-N-succinyl chitosan nanogels containing loteprednol etabonate: A new system for therapeutic effect enhancement via controlled drug delivery. Eur. J. Med. Chem. 2015, 102, 132–142.
