Long non-coding RNAs (lncRNAs) represent crucial transcriptional and post-transcriptional gene regulators during antimicrobial responses in the host innate immune system. Studies have shown that lncRNAs are expressed in a highly tissue- and cell-specific- manner and are involved in the differentiation and function of innate immune cells, as well as inflammatory and antiviral processes, through versatile molecular mechanisms. These lncRNAs function via the interactions with DNA, RNA, or protein in either cis or trans pattern, relying on their specific sequences or their transcriptions and processing. The dysregulation of lncRNA function is associated with various human non-infectious diseases, such as inflammatory bowel disease, cardiovascular diseases, and diabetes mellitus. Here, we provide an overview of the regulation and mechanisms of lncRNA function in the development and differentiation of innate immune cells, and during the activation or repression of innate immune responses. These elucidations might be beneficial for the development of therapeutic strategies targeting inflammatory and innate immune-mediated diseases.
- long non-coding RNA
- transcriptional regulation
- inflammation
- innate immunity
- innate immune cells
1. Introduction
The innate immune system is equipped with an arsenal of strategies to withstand infectious threats and maintain the normal activities and metabolism of the body. Activation of the innate immune system represents an immediate and initial response against pathogens and endows the body with the ability to repair and restore damaged tissue. Macrophages, dendritic cells, and granulocytes are important innate immune cells that participate in the immune response by sensing specific pathogen-associated molecular patterns (PAMPs) through their germline-encoded pattern recognition receptors (PRRs) [1]. The recognition of PAMPs by PRRs triggers an array of activation of intracellular signaling cascades, including adaptors, kinases, and transcription factors, leading to the expression of proinflammatory cytokines or antimicrobial genes [2]. However, activation of the inflammatory process can be a double-edged sword: although it is a crucial part of pathogen elimination, prolonged activation of these complex pathways might lead to tissue damage and diseases including cancer, cardiovascular diseases, and rheumatoid arthritis [2]. Thus, unsurprisingly, all of the aspects involved in inflammatory signaling pathways are tightly regulated at both the transcriptional and post-transcriptional levels [3].
Long non-coding RNAs (lncRNAs), which are defined as transcripts longer than 200 nucleotides and lacking protein-coding potential, represent the largest group of non-coding RNAs transcribed from the genome [4]. In the most recent LncRBase V.2 database release, 241,562 and 178,336 lncRNA transcripts were defined from the human and mouse genomes, respectively [5]. Advances in high-throughput technologies led to rapidly growing data accumulation in the identification of characteristics and functions of lncRNAs, which greatly expanded our understanding of the intricate and intriguing lncRNA biology. The gene-regulatory functions of lncRNAs not only depend on their specific transcript sequences but also on their own transcriptional process, which might account for the lower conservation of exon sequences but higher conservation of promoter regions in lncRNAs compared with that in protein-coding genes [6][7]. LncRNAs can regulate gene expression via multiple mechanisms at both the transcriptional and post-transcriptional levels [8]. Recent studies found that lncRNAs extensively participate in a series of biological and physiological processes, such as chromatin remodeling, cell cycle and proliferation, and metabolic homeostasis [9][10]. Moreover, lncRNA acts as a key regulator of innate immune responses and inflammation by activating various signal-dependent chromatin-modifying factors, transcription factors, and transcriptional coregulators [11]. However, the exact mechanisms of lncRNA bioactivities in innate immunity are still not completely clear.
Herein, we review the recent advances in illustration of the functions and mechanisms of lncRNAs in innate immune cell development and innate immune responses. In addition, we also discuss the important biological characteristics of lncRNAs, including their transcription, alternative splicing, cellular localization, and conservation. Despite the limited number of studies at present, we envision and believe that more intriguing lncRNAs and their biological functions will be exploited in the innate immune system in the near future.
2.Long Non-Coding RNAs
According to the Encyclopedia of DNA Elements (ENCODE) consortium (2012), the vast majority of the mammalian genome is transcribed [12][13]; nonetheless, only a small proportion (about 2%) of the genome is composed of genes that encode proteins, while the majority is transcribed as non-coding RNAs [7][14]. GENCODE represents the gene set of the ENCODE project and its most recent release (updated in 2021, https://www.gencodegenes.org (accessed on 5 May 2021)) indicates that there are 17,944 and 13,188 lncRNAs present in the human and mouse genomes, respectively (Figure 1A).
2.1. Classification of Long Non-coding RNAs
In general, non-coding RNAs are categorized into lncRNAs and short non-coding RNAs according to a sequence length cutoff of 200 nucleotides. Based on their genomic location relative to enhancer elements or protein-coding loci, lncRNAs are further classified as enhancer RNAs (eRNAs), intronic lncRNAs, long intergenic non-coding RNAs (lincRNA), sense and antisense lncRNAs (Figure 1B). LncRNAs are often transcribed from either strand, within or outside non-protein-coding loci by RNA polymerase II (Pol II), and, like message RNAs (mRNAs), are capped, spliced, and polyadenylated [8][15]. LincRNAs refer to lncRNAs that are strictly intergenic and have no overlap with known protein-coding genes, while all other forms of lncRNAs overlapped with mRNA to varying degrees. eRNAs are particularly intriguing because they can be transcribed bidirectionally and capped from active enhancers but not spliced or polyadenylated [16]; however, some eRNA-liked lncRNAs transcribed from regulatory elements can be polyadenylated and unidirectional [17]. Antisense lncRNAs (Figure 1B) seem to be the primary lncRNA subtype, as it has been reported that over 70% of murine genomic loci are transcribed as antisense lncRNAs [18]. Although it was originally professed that lncRNAs are unstable, which is only true for a minority of lncRNAs, most can be stabilized through polyadenylation [19], and non-polyadenylated lncRNAs can also be stabilized depending on their secondary structures, such as hairpin motifs and triple-helical structures [20][21].
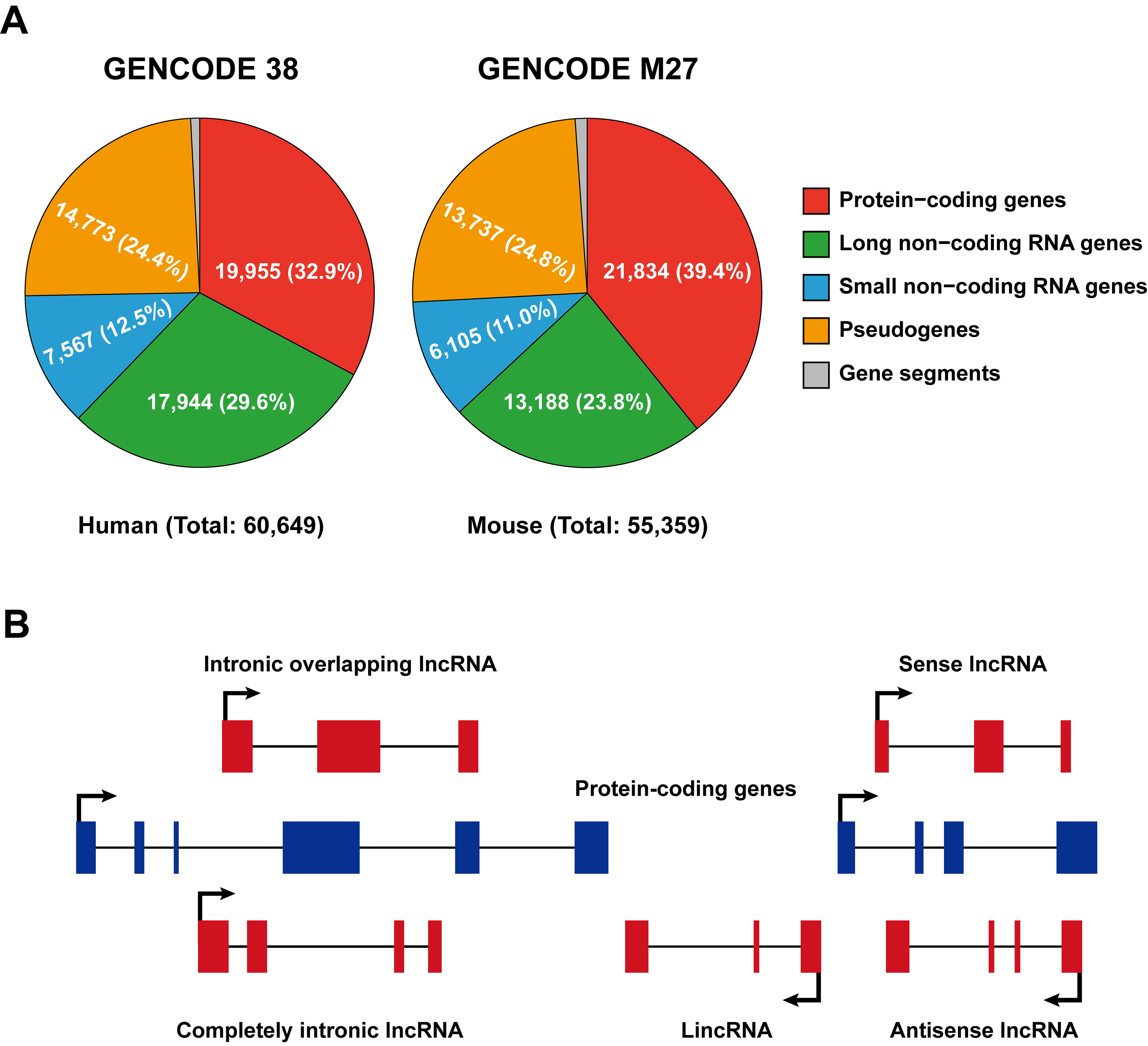
Figure 1. Classification of long non-coding RNAs. (A) Pie charts illustrating numbers and percentages of protein- and non-coding genes in the human and mouse genomes (released from GENCODE, 2021 update; https://www.gencodegenes.org (accessed on 5 May 2021)). (B) Classification of lncRNAs based on their localization with respect to genomic protein-coding genes. Sense/antisense lncRNAs are transcribed in the same/opposite direction as protein-coding genes and overlap at least one coding exon; completely intronic lncRNAs are transcribed from the intron of a protein-coding gene; intronic overlapping lncRNAs contain intronic sequences; lincRNAs are transcribed from regions between two protein-coding gene loci.
2.2. Transcription and Degradation of Long Non-Coding RNAs
Although lncRNAs and mRNAs have several similarities in their transcriptional process as mentioned above, large transcriptional differences have been identified between these two types of RNAs. Mammalian native elongation transcript sequencing (mNET-seq) data reveal that lincRNAs and mRNAs are transcribed by Pol II that differ in the phosphorylation state of the C-terminal domain (CTD). Usage of the Pol II isoform, which lacks phospho-CTD features associated with co-transcriptional splicing, and 3′ cleavage and polyadenylation, allows for lincRNAs with lower levels of co-transcriptional splicing and inefficient polyadenylation compared with pre-mRNAs [22]. Moreover, different from pre-mRNAs, lincRNAs are mostly restricted to chromatin, partially due to interaction with the U1 snRNP. These lincRNA are degraded by the nuclear RNA exosome, resulting in few lincRNAs being detected in cytoplasm [23]. However, the reasons that chromatin-associated lincRNAs rapidly degrade after transcription remain elusive.
In addition to the rapid post-transcriptional degradation, and low expression of lncRNAs [24], as well as their tissue- and cell-type specificity, they are directly associated with DNA methylation and histone modification. Mammalian promoters can be categorized into two types according to their CpG dinucleotide content: high CpG (HCG) and low CpG (LCG) [25]. The HCG class of promoters is hypomethylated, while the LCG class of promoters is hypermethylated, and the latter generally repress gene transcription. In the mammalian genome, a large proportion of lncRNAs are transcribed from the LCG class of promoters, of which only 6.5% are marked by histone H3K4me3, associated with higher transcription activity, resulting in low expression levels for lncRNAs. Moreover, the abundance of CpG dinucleotide usually has a negative correlation with the potential for chromosome condensation, indicating that lncRNA promoters may be highly condensed and unsuitable for transcription [26]. Moreover, enrichment of transcription factor binding sites [[27] and specific DNA sequence (e.g., CCG and CGG repeats) in lncRNA loci [28] are significantly correlated with lncRNA expression. In addition, miRNA-dependent regulation of promoter methylation leads to the complexity of dynamic expression of lncRNAs during diseases [29].
Considering the short history of lncRNA studies, more detailed transcriptional features of lncRNAs should be investigated further—for example, researchers must establish whether lncRNA transcription depends on other regulatory non-coding RNAs.
2.3. Alternative Splicing of Long Non-Coding RNAs
Similar to protein-coding mRNAs, the vast majority of lncRNAs undergo extensive alternative splicing, which greatly increases their potential number of isoforms [30]. Overall, lncRNAs are spliced less efficiently than mRNAs, and the splicing frequency of specific introns in lncRNAs is usually more variable compared with that of mRNA introns [31], leading to substantially more alternative splicing isoforms of lncRNAs. Moreover, different from mRNAs, there is no need to maintain open reading frames (ORFs), which may allow the spliceosome to explore the full spectrum of exon combination, resulting in a high diversity of lncRNA isoforms [30]. In addition, recent reports have also proved that lncRNAs can act as the precursors of miRNAs. The exon alternative splicing of lncRNAs can produce important conserved miRNAs [32]. The alternative splicing of either protein-coding or non-coding RNAs is regulated by a comprehensive list of cis-regulatory elements and trans-regulatory factors. These cis-regulatory elements contain intron-splicing enhancers (ISEs) and silencers (ISSs), and exon-splicing enhancers (ESEs) and silencers (ESSs), while trans-acting factors include Ser/Arg-rich (SR) proteins and heterogeneous nuclear ribonucleoproteins (hnRNPs), which function via binding to cis-acting sequences [33][34]. SR proteins are generally considered positive splicing regulators and promote exon inclusion by recruiting U1 snRNP and U2 auxiliary factors to exons; in contrast, hnRNPs, including hnRNP A/B and PTB, are viewed as negative for cis-acting elements [34]. These extensive alternative splicings, as a complex and overlooked aspect of lncRNAs, might further diversify the potential biological function of lncRNAs, along with newly identified lncRNA partners or localization patterns, due to the specific exon involvement; however, this is largely unknown.
2.4. Conservation and Secondary Structure of Long Non-Coding RNAs
At the sequence level, researchers found that lncRNAs are, overall, much less conserved compared with protein-coding genes [35][36]. The poor sequence conservation and low abundance of lncRNAs initially led some researchers to suggest that most lncRNAs may represent transcriptional “noise” and have little biological significance [37]. Nevertheless, some studies showed that >85% of human GENCODE lncRNAs can be dated back to the divergence of placental mammals according to the conserved splice sites [38]. Despite the fast turnover of exon/intron structures that was observed, the exons show higher conservation than introns in human lncRNAs[7].
Secondary structure is one of the crucial factors determining the function of lncRNAs, as supported by several classical functionally characterized lncRNAs in the previous analyses. For instance, the functions of MEG3 for the activation of p53 signaling and suppression of tumor cell growth can be attributed to a secondary folding motif, identified using an RNA secondary structure prediction program, Mfold [39]. It has also been shown that MALAT1 requires an intact U-rich stem loop duplex-triplex and A-rich tract to maintain its RNA stabilization activity [20]. Overall, wet experiments suggest that lncRNAs exhibit a higher degree of secondary folding than that predicted by algorithms, despite the fact that lncRNAs seem to be less structured than mRNAs [40]. These specific secondary structures may play a decisive role in lncRNA bioactivities.
2.5. Subcellular Localization of Long Non-Coding RNAs
The subcellular localization of lncRNAs is very important, as it provides critical information for the understanding and prediction of, as well as investments in, non-coding action patterns, including associated molecules, post- or co-transcriptional regulatory modifications, and external stimuli directly affecting lncRNA functions. Unlike mRNAs, which are translated into proteins in the cytoplasm regardless of their functions, lncRNAs must localize where they play specific roles. In accordance with this, studies indicate that nuclear lncRNAs are more abundant than cytoplasmic ones probably due to their main functions in nuclear architecture organization [41][42][43], although the number of cytoplasmic lncRNAs is gradually expanding. However, nuclear lncRNAs seem to be more instable due to their low expression and the involvement of unstable transcripts, such as upstream promoter transcripts. This might reflect their specific function in gene expression, as well as transcriptional or post-transcriptional regulation of gene expression [19]. Several RNA sequence motifs have been identified as responsible for nuclear localization, such as C-rich motifs outside Alu-like elements in the sequence of some lncRNAs [44]. The repeat E motif was also found to be involved in the localization of lncRNA Xist [[45]. In addition to their own characteristics, including genomic and subcellular localization, GC percentage and splicing, the instability of nuclear lncRNAs can also be regulated by the poly (A) binding protein PABPN1, which promotes poly (A)-polymerase (PAPα/β)-dependent hyperadenylation and subsequent decay [46].
Besides nuclei, studies also have begun to interrogate the localization of lncRNAs to specific macromolecular structures or organelles. This localization of RNA has been largely studied using fractionation-based methods combined with RNA-seq and fluorescence in situ hybridization (FISH) [47][48]. APEX is an engineered peroxidase and can catalyze biotin-phenol and hydrogen peroxide to form biotin-phenoxyl radicals. These radicals can then diffuse outward and covalently biotinylate the adjacent endogenous proteins, but not the distal proteins, because of their extremely short half-life. Therefore, a method in living cells that combines an engineered APEX that targets the cellular compartment of interest [49][50] with RNA immunoprecipitation (RIP) has allowed for the identification and quantification of RNAs localized in varieties of subcellular compartments, including the nucleus, cytosol, mitochondrial matrix, and endoplasmic reticulum (ER) [41]. Another RNA aptamers (consisting of Tat peptide and trans-activation response (TAR) element) and fluorogenic proteins system also provided a pipeline to visualize RNA localization in living cells [51]. In this system, a bifunctional peptide (termed “tDeg”), containing a Tat peptide and degron sequence, is fused to the fluorogenic protein. The RNA aptamers (termed “Pepper”) inserted into the RNA of interest can bind to the Tat peptide, preventing degron from recruiting proteasome and stabilizing the fluorogenic protein. Thus, the RNA of interest can be detected by the fluorescence signal. Based on these established methods, a small portion of organelle-related lncRNAs have been identified and further functionally characterized. Moreover, cellular localization of lncRNAs can be predicted using a publicly available web server iLoc-LncRNA (http://lin-group.cn/server/iLoc-LncRNA (accessed on 15 Dec 2018)) [52], which can be considered as the first step for researchers attempting to predict the localization of their candidates according to their sequences.
3. Long Non-Coding RNAs Function as Transcriptional Regulators
Although the biological functions of lncRNAs are just starting to be studied and understood, it has been known that lncRNAs play critical roles in almost every biological process mainly through three different patterns: the lncRNA molecule itself is functional depending on its specific sequence; the process of lncRNA transcription, rather than the lncRNA molecule itself, has a function; lncRNA functions as proxy signals for active cis-regulatory elements (Figure 2) [53]. During these processes, it has been proposed that lncRNAs can exert regulatory roles, either in cis or in trans, by serving as molecular signals, decoys, guides, and scaffolds, through interacting with DNA, RNA, or proteins [54]. However, given their exquisite cell-type-specific expression pattern and poor sequence conservation, it remains to be understood whether lncRNAs have new action patterns besides these three mechanisms
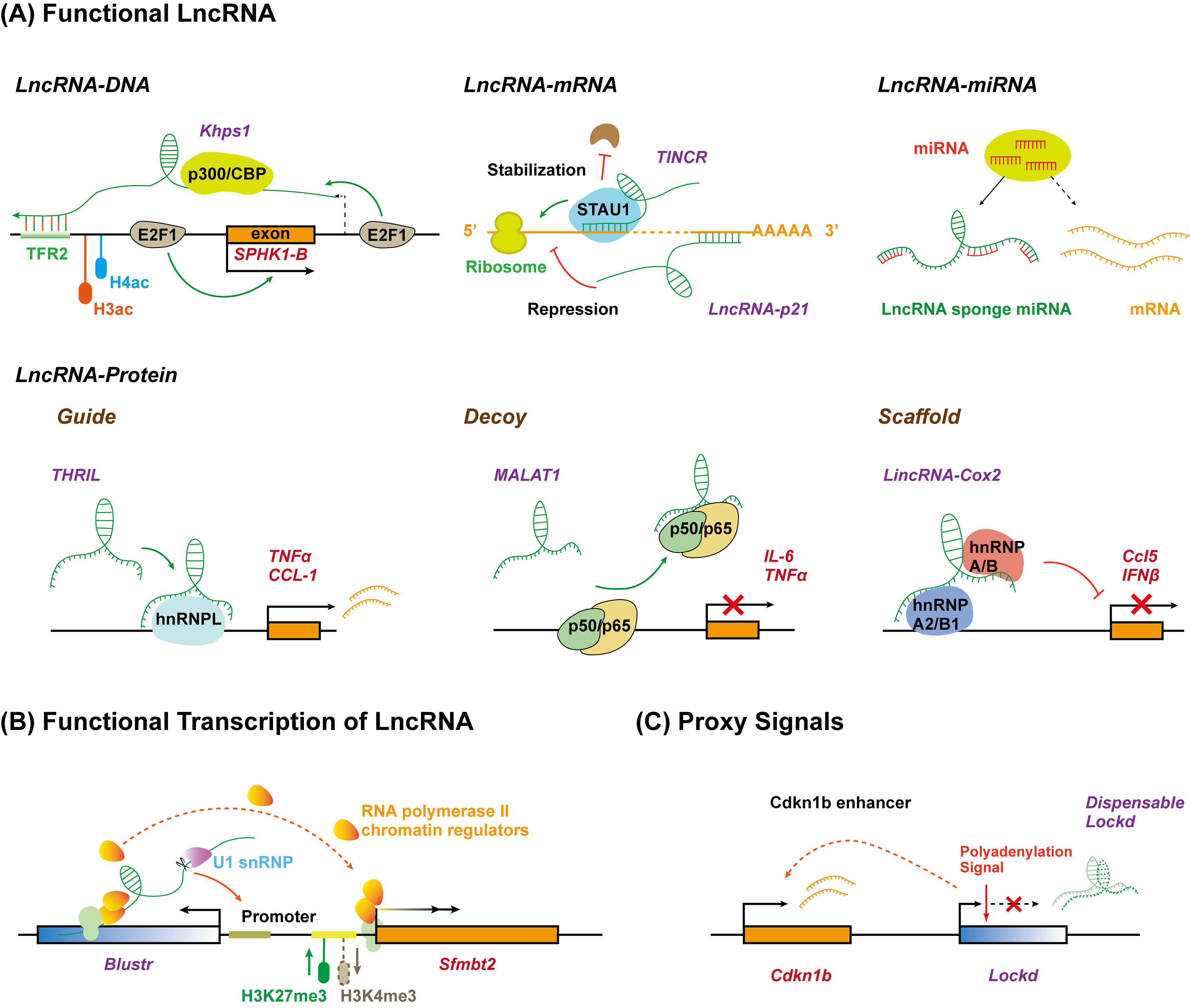 Figure 2. Long non-coding RNAs function as versatile transcriptional regulators in three different manners. (A) LncRNA molecule is functional due to its specific sequence interacting with DNA, RNA, or protein. LncRNA (Khps1) tethers to DNA via formatting triplex with specific sequence and guides chromatin regulators to target gene (SPHK1-B) promoter. LncRNA interacts with target mRNA by base-pairing to repress (lncRNA-p21) or enhance (TINCR) translation. LncRNA sponges miRNA to inhibit degradation and translation repression of mRNA. LncRNA interacts with specific proteins to regulate target gene expression by acting as a guide (THRIL), decoy (MALAT1), or scaffold (lincRNA-Cox2). (B) The act of lncRNA transcription is functional. The transcription and promoter-proximal splicing process of lncRNA (Blustr) alter the chromatin state and ensure RNA polymerase elongation at the promoter of target gene (Sfmbt2). (C) LncRNA acts as proxy signal for cis-regulatory elements. The 5′region or promoter of lncRNA (Lockd) contains an enhancer for the neighboring genes (Cdkn1b). TFR, triplex-forming regions.
Figure 2. Long non-coding RNAs function as versatile transcriptional regulators in three different manners. (A) LncRNA molecule is functional due to its specific sequence interacting with DNA, RNA, or protein. LncRNA (Khps1) tethers to DNA via formatting triplex with specific sequence and guides chromatin regulators to target gene (SPHK1-B) promoter. LncRNA interacts with target mRNA by base-pairing to repress (lncRNA-p21) or enhance (TINCR) translation. LncRNA sponges miRNA to inhibit degradation and translation repression of mRNA. LncRNA interacts with specific proteins to regulate target gene expression by acting as a guide (THRIL), decoy (MALAT1), or scaffold (lincRNA-Cox2). (B) The act of lncRNA transcription is functional. The transcription and promoter-proximal splicing process of lncRNA (Blustr) alter the chromatin state and ensure RNA polymerase elongation at the promoter of target gene (Sfmbt2). (C) LncRNA acts as proxy signal for cis-regulatory elements. The 5′region or promoter of lncRNA (Lockd) contains an enhancer for the neighboring genes (Cdkn1b). TFR, triplex-forming regions.
3.1. Functional Long Non-Coding RNA Molecules
The most common method through which to study the function of lncRNAs is to characterize the direct biological activity of the lncRNA molecule itself. Most lncRNAs discovered to date modulate transcription by binding to the target DNA in cis (for neighboring genes) or trans (for distal genes) through the recognition of specific chromatin features (Figure 2A). They can interact with single-stranded or double-stranded DNA by forming RNA–DNA hybrid duplex or RNA–DNA triplex structures through Watson–Crick or Hoogsteen hydrogen bonding [54][55]. For example, a lncRNA, Khps1, can anchor to proto-oncogene SPHK1 promoter by forming a DNA–RNA triplex with a homopurine stretch upstream of the transcription start site. Then, Khps1 recruits the histone acetyltransferase p300/CBP to the SPHK1 promoter, changing the chromatin structure and facilitating SPHK1 transcription in cis by ensuring the binding of transcription factor E2F1 [56]. CTCF is one highly conserved zinc finger protein and can coordinate chromatin structures to regulate gene expression [57]. LncRNA CCAT1-L localizes at the transcription site, spatially close to the MYC oncogene, and maintains the chromatin looping between the MYC promoter and its enhancers in coordination with CTCF, thus, enhancing MYC transcription in cis [58]. HOTAIR transcribed from the HOXC locus has been demonstrated to interact with methyltransferase Polycomb repressor complex 2 (PRC2) subunits (including Ezh2 and Suz12) [59] and functions as a bridge by recruiting PRC2 to the gene loci to promote target gene silencing through a complex array of post-translational modifications of histones in trans [60]. These studies also indicated that lncRNAs can regulate gene transcription by acting as a bridge between chromatin-modifying proteins and chromatin modification elements. The heterogeneous nuclear ribonucleoproteins (hnRNPs) are predominant nuclear RNA-binding proteins that form complexes with RNA polymerase II transcripts, which function in the transcription, processing, and translation of mRNA [61]. A growing body of research has indicated that lncRNAs, such as lincRNA-p21 (hnRNP-K) [62], lncRNA-ITPF (hnRNP-L) [63], LincRNA-Cox2 (hnRNP-A/B) [11], and lncRNA ST3GAL6-AS1 (hnRNPA2B1)[64] can recruit transcriptional machinery to the promoters of target genes via association with hnRNPs [62].
In addition to regulating gene transcription, lncRNAs containing miRNA response elements (MREs) can regulate protein-coding mRNAs harboring the same MREs by acting as competing endogenous RNAs (ceRNAs) or natural microRNA sponges at the post-transcriptional level [65][66]. The competitive binding of these ceRNAs to the seed region of the shared miRNAs results in derepression of other RNA transcripts that contain the same MREs [66]. Extensive transcriptome data have revealed that a large repositories of lncRNA/miRNA pairs, such as MALAT1-miR-181c-5p/miR-125b/miR-146a/miR-199b [67][68][69][70], lincRNA-Cox2-let-7a/miR-150-5p [71][72], RP11-86H7.1-miR-9-5p [73], SNHG5-miR-132 [74], play an important role in inflammation and innate immunity via the ceRNA regulatory network.
Future studies on lncRNA structures and motifs will contribute to a better understanding of the mechanisms by which lncRNAs interact with specific DNA, RNA, and proteins, as well as how lncRNAs localize at specific sites.
3.2. Functional Roles of the Act of Long Non-Coding RNA Transcription
In some contexts, the biogenesis process for a lncRNA but not the lncRNA itself can impact the expression of nearby genes (in a cis pattern), because the process of transcription or splicing of lncRNAs may recruit specific protein factors (e.g., transcription factors, repressor proteins, and polymerase) or remodel nucleosomes, which regulates gene transcription [75][76]. Genetic manipulation in mouse cell lines found that five genomic loci that produce lncRNAs influence the expression of the neighboring gene in cis and, intriguingly, all of these effects do not require the specific lncRNA transcripts themselves, while they do involve the general processes associated with their production, including the enhancer-like function of their promoters, the transcription process, and/or the splicing of these transcripts (Figure 2B) [77]. Deletion of the promoter of lncRNA Bendr (linc1536) decreased the expression of the nearby protein-coding gene Bend4 by 57%; however, the effects require neither a mature nor a significant amount of the Bendr transcript [77]. In addition, an increase in the length of lncRNA Blustr (linc1319)-transcribed region by engineered pAS insertions promoted the activation of Sfmbt2, located 5 kB upstream, independent of any specific sequence elements in mature Blustr. Moreover, the first 5′ splicing site of Blustr plays a vital role in activating Sfmbt2 transcription, probably because the splicing event leads to the recruitment of transcriptional machinery acting on the nearby Sfmbt2 promoter [77].
Although it is becoming clearer that the production process of lncRNAs influences neighboring gene transcription, how transcription and splicing across lncRNA loci recruit the vital regulatory elements or change the dynamic of chromatin to coordinate gene expression in cis remains to be illustrated.
3.3. Long Non-Coding RNAs Act as Proxy Signals for Cis-Regulatory Elements
In addition to the production process of lncRNA transcripts, certain conserved lncRNA promoters may regulate transcription of the adjacent genes as cis-acting enhancer elements (Figure 2C)[78]. In this case, lncRNA transcripts are non-functional byproducts marking the regulatory activity of their promoters. For example, the deletion rather than truncation of lncRNA Lockd impairs the transcription of its upstream coding gene Cdkn1b, due to the enhancer-like DNA elements within the Lockd promoter [79]. Therefore, the transcription of lncRNAs might act as proxy signals of the activity of crucial DNA regulatory elements.
The prevalence of the three functional mechanisms mentioned above suggests two patterns for the evolutionary selection of lncRNAs: one is restricted to the RNA sequence while in another, functional cis-regulatory elements but not the sequence of lncRNAs are implicated [24][35]. This raises the possibility that, despite the limited sequence conservation, some lncRNAs have conserved functions across species.
4. Long Non-Coding RNAs Function in Innate Immunity
In biological immune responses, the innate immune system serves as the initial defense against foreign and harmful substances. Both professional innate immune cells, including macrophages, mast cells, natural killer (NK) cells, neutrophils, eosinophils, basophils, and dendritic cells (DCs), and nonprofessional innate immune cells, such as endothelial cells, and fibroblasts [2], undergo immediate rapid changes in gene expression and regulation programs to respond to pathogenic invasion, tissue damage, stress, and metabolic dysregulation [80]. Although pathogens can evolve rapidly, the innate immune system can always detect the invading pathogens and common biologic consequences of infection relying on a limited repertoire of receptors. Innate immune cells have evolved to target conserved microbial components that are shared by most pathogens to compensate for the limited number of receptors. To enlarge cellular defenses, the innate immune system also contains many humoral components, including well-characterized components, such as C-reactive protein, complement proteins, and lipopolysaccharide (LPS) binding protein, and less-well-studied antimicrobial peptide components.
Given their role in mediating gene transcription and translation, a growing body of discoveries has revealed that lncRNAs are excellent candidates for the regulation of the mammalian innate immune processes, including the clearance of bacterial and viral infection, host inflammatory responses, and development of manifold innate immune-mediated diseases, in both positive and negative patterns [81]. These findings have served as an impetus for a further and thorough exploration of how lncRNAs regulate the innate response as well as the sophisticated immune cell development. We will focus on lncRNAs that are recently identified and widely studied and review their functions and underlying mechanisms according to their dependent signaling molecules and patterns of action.
4.1. Long Non-Coding RNAs in the Development of Innate Immune Cells
In recent years, lncRNAs have merged as regulators of somatic cell differentiation in tissues ranging from epidermal to adipose tissues [82][83], as well as osteogenic differentiation of mesenchymal stem cells [84], while their biology and function in the development, differentiation, and maturation of professional innate immune cells are only beginning to be explored. Innate immune cells are generated from hematopoietic stem cells (HSCs) and consist of myeloid cells derived from mononuclear phagocytes (e.g., macrophages, differentiated from blood monocytes) and polymorphonuclear phagocytes (e.g., granulocytes), and lymphoid lineage cell-derived NK cells [85]. While the adaptive immune system mainly includes T and B lymphocytes, innate immune cells are vital in the immune system because they support the functions of the adaptive immune system, depending on the production of cytokines and the antigen-presenting function [86].
4.1.1. Macrophages
Macrophages belong to the mononuclear phagocyte system, defined by their origin from bone-marrow-derived cells, and their phagocytosis, cytokine secretion, and antigen presentation abilities. Cells of the mononuclear phagocyte system have a great capacity to specialize, particularly during inflammatory response, where monocytes are recruited into the tissues and differentiate into macrophages. Macrophages are vital cells for innate immune sensing, accomplished by Toll-like receptors (TLRs) on their surface, and are considered as the first line of the host innate immune system [87]. It has been found that in the process of monocyte/macrophage differentiation of THP-1 cells and CD34+ HSPCs, lnc-MC (Table 1) promotes the differentiation process by sequestering miR-199a-5p and releasing the expression of activin A receptor type 1B (ACVR1B), an important regulator of monocyte/macrophage differentiation [88]. Overexpression of PBOV1 in THP-1 cells results in their differentiation into macrophages, and an RNA IP assay showed that lncRNA NTT could upregulate PBOV1 expression by interacting with hnRNP-U binding to the promoter of PBOV1 [89].
Table 1. Long non-coding RNAs in the development and polarization of innate immune cells.
|
LncRNA |
Target Genes |
Functional |
Mechanism |
Reference |
|
Lnc-MC |
miR-199a-5p |
Promotes macrophage differentiation |
Releases ACVR1B |
[88] |
|
NTT |
PBOV1 |
Promotes macrophage differentiation |
Recruits hnRNP-U to PBOV1 promoter |
[89] |
|
LincRNA-Cox2 |
NF-κB-mediated cytokines |
Inhibits M2 polarization |
- |
[90] |
|
GAS5 |
miR-455-5p |
Promotes M1 polarization from M2 |
Release SOCS3 |
[91] |
|
MIR-155HG |
Proinflammatory cytokines |
Induces M1 polarization |
- |
[92] |
|
Mirt2 |
TRAF6 |
Promotes M2 polarization |
Suppresses NF-κB and MAPK pathway |
[93] |
|
LncRNA-MM2P |
STAT6 |
Promotes M2 polarization |
Increases phosphorylation of STAT6 |
[94] |
|
PTPRE-AS1 |
PTPRE |
Inhibits M2 activation |
Recruits WDR5 to PTPRE promoter |
[95] |
|
Lnc-DC |
STAT3 |
Promotes DCs differentiation |
Prevents Y705 dephosphorylation of STAT3 by SHP1 |
[96] |
|
MALAT1 |
miR-155 |
Induces tolerogenic DCs |
Releases DC-SIGH and IL-10 |
[97] |
|
HOTAIRM1 |
HOXA cluster, CD11b and CD18 |
Promotes granulocyte differentiation and maturation |
- |
[98] |
|
Lnc-CD56 |
CD56 |
Promotes CD56 NK cell development |
- |
[99] |
|
GAS5 |
miR-544 |
Enhances CD107a+ NK cells and its cytotoxicity |
Upregulates RUNX3 as a sponge |
[100] |
|
Linc-EPHA6-1 |
has-miR-4885-5p |
Promotes cytotoxicity of NK cells |
Upregulates NKp46 expression as a sponge |
[101] |
In response to various pathogen- and self-local-environment-derived stimuli, macrophages exhibit a strong phenotypic and functional plasticity and complexity [102], where M1 (classically activated macrophages) and M2 (alternatively activated macrophages) represent two extreme macrophage subtypes in vitro [103]. Functionally, M1 macrophages that are activated by bacterial LPS and interferon-γ (IFN-γ) produce abundant amounts of proinflammatory cytokines (such as TNF-α, NO, IL-1, IL-12, and IL-23) or reactive oxygen species (ROS) to kill pathogens and promote Th1 immune response. TLR-triggered NF-κB is one of the well-studied pathways that participates in the polarization of macrophages to M1 phenotype [104]. Transcriptome analysis has shown that the expression levels of numerous lncRNAs are altered in macrophages upon stimulation of TLR ligands including LPS. Of note, several LPS-regulated lncRNAs, such as lncRNA-NfκB2 and lncRNA-Rel, are located near to proinflammatory protein-coding genes, indicating their potential role in regulating M1 macrophage polarization [105].
However, in the presence of granulocyte macrophage colony stimulating factor (GM-CSF), IL-4, IL-10, IL-13, or immune complexes (ICs) together with either TLR or IL-1R ligands, macrophages tend to polarize into M2 subtypes, which subsequently leads to an anti-inflammatory Th2 response, thus, enhancing tissue repair and remodeling [106]. Microarray analysis found 264 upregulated and 289 downregulated lncRNAs in IL-4 induced M2 macrophages. Additionally, PTPRE-AS1, one of these potently enhanced lncRNAs, acts as a repressor of M2 activation by activating PTPRE through the recruitment of WDR5 to the PTPRE promoter [95]. As one of the most highly induced lncRNAs in macrophages by TLR activation, lincRNA-Cox2, located downstream of protein-coding Cox2, is required for the NF-κB-mediated transcription of proinflammatory genes [11], whereas it inhibits M2 polarization [90]. LncRNA growth-arrest-specific 5 (GAS5) shifts macrophages toward the M1 subtype from the M2 subtype by acting as a miR-455-5p ceRNA regulator that promotes SOCS3 expression during childhood pneumonia [91]. Similar to GAS5, MIR-155HG induces M1 macrophage polarization, whereas it impedes M2 polarization, albeit through an unknown underlying mechanism [92]. In contrast, lncRNA Mirt2 acts as a negative regulator of LPS-activated inflammatory response by suppressing NF-κB and MAPK pathways in macrophages [93], and lncRNA-MM2P promotes cytokine-stimulated M2 polarization by enhancing signal transducer and activator of transcription 6 (STAT6) phosphorylation [94]. Despite the fact that the lncRNA has been shown to be a key regulator of macrophage polarization, there is still substantial room for research, especially in vivo.
4.1.2. Dendritic Cells
In the same way as macrophages, DCs originate from the mononuclear phagocyte system, and bridge the innate and adaptive arms of the immune system by acting as the primary antigen-presenting cells (APCs) for T lymphocytes [107]. DCs are classified into two subtypes: conventional DCs (cDCs) that function as APCs, and plasmacytoid DCs (pDCs) that produce copious levels of the type I IFN against viral and bacterial infections [108]. RNA-seq analysis at different stages of monocyte differentiation into DCs identified a cohort of regulated lncRNAs involved in DC maturation. A lncRNA that is exclusively expressed in human DCs, named lnc-DC, is vital for DC differentiation from both human monocytes and mouse bone marrow cells through controlling the expression of DC markers CD40, CD80, CD86, and HLA-DR by binding to the transcription factor STAT3 [96]. In addition, lnc-DC deficient DCs failed to take up antigens and induce allogeneic CD4+ T cell proliferation and cytokine production [96]. LncRNA MALAT1 can induce tolerogenic DCs via the prevention of miRNA-155 targeting of DC-SIGH and IL-10 as a sponge [97].
4.1.3. Granulocytes
The HOXA gene cluster is a homeotic gene that encodes a family of transcription factors that participate in the establishment and maintenance of cellular identity in embryogenesis [109]. The intergenic non-coding transcript HOX antisense intergenic RNA myeloid 1 (HOTAIRM1) located between the HOXA1 and HOXA2 genes has been demonstrated to be associated with granulocytic differentiation and maturation [98]. HOTAIRM1 expression is specific to the myeloid lineage and is upregulated during the retinoic acid (RA)-promoted granulocytic differentiation of NB4 promyelocytic leukemia and human normal hematopoietic cells. In addition, the shRNA-mediated knockdown of HOTAIRM1 attenuated the transcriptional induction of its neighboring genes at the 3′ end of the HOXA cluster and impeded the transcription of genes encoding β2 integrins CD11b and CD18. Moreover, the association of HOXA genes with the transcriptional regulation of normal hematopoiesis [110] and acute myeloid leukemia [111] indicates that HOTAIRM1 may additionally play functional roles in myelopoiesis via regulating HOXA expression in cis.
4.1.4. Natural Killer Cells
Different from macrophages, DCs, and granulocytes of myeloid origin, NK cells featuring CD3-negative and CD56-positive surface markers are innate lymphoid cells with cytotoxic effects[112] and produce various cytokines in response to bacterial, viral, and parasitic infections [80]. Recent studies demonstrated that lncRNAs play important roles in the development and function of NK cells. An example of a prototypical lncRNA with cis regulatory function in NK cells is lnc-CD56 [99], which is highly expressed in human CD56bright NK cells, and has a superior ability to produce proinflammatory cytokines in comparison with more cytotoxic CD56dim NK cells [113]. Knockdown of lnc-CD56 reduced CD56 expression and decreased mature CD56bright NK cell content, demonstrating the requirement of lnc-CD56 for CD56 maintenance during NK cell development [99]. Another lncRNA GAS5 was found to be downregulated in the NK cells of liver cancer patients, which causes a reduction in IFN-γ production, a decrease in the percentage of CD107a+ NK cells and the impaired cytotoxicity of NK cells, attributed to RUNX3 upregulation by sponging miR-544 [100]. Recent research has also showed that exosomal linc-EPHA6-1, induced by IFN-β, can promote NKp46 expression and cytotoxicity of NK cells through its interaction with has-miR-4885-5p [101].
Together, these studies demonstrate the importance of lncRNAs in controlling the development of innate immune cells. Nevertheless, more research should be carried out to decipher the mechanisms underlying the roles of lncRNA involved in innate immune cell differentiation and polarization, as well as their functions.
4.2. Long Non-Coding RNAs Function in Host Inflammatory Response Triggered by the Innate Immune System
4.2.1. Inflammatory Signaling Triggered by PAMPs and DAMPs
The innate immune response is initiated by the binding of microbial structures, termed pathogen-associated molecular patterns (PAMPs), to PRRs on the surface of innate immune cells [2]. The second defensive approach used by the innate immune system is the to detection of an immunological danger signal in the form of damage-associated molecular pattern molecules (DAMPs), such as high-mobility group box 1 protein, heat shock proteins, and uric acid, that are released from infected or damaged host cells [114]. Based on their protein domain-structure, PRR families are classified into four classes: Toll-like receptors (TLRs), C-type lectin receptors (CLRs), Retinoic acid-inducible gene (RIG)-I-like receptors (RLRs), and NOD-like receptors (NLRs) [2]. Different PRR family members can recognize diverse pathogen structures ranging from bacterial and viral nucleic acids, such as unmethylated CpG DNA (TLR9), dsRNA (e.g., TLR3, MDA5, Rig-I), or cytosolic DNA (cGAS), to bacterial cell wall components, such as bacterial lipoproteins (TLR2), lipopolysaccharides (TLR4), and peptidoglycans (NLRs) [2][115]. When PRRs sense the presence of PAMPs or DAMPs, the transcription of genes encoding proinflammatory cytokines (e.g., TNF-α, IL-1, IL-6), chemokines (e.g., CCL2, CXCL8), interferons, antimicrobial proteins, or proteins involved in the modulation of PRR signaling are upregulated by the activation of master-transcription factors during the immune response, such as NF-κB, MAPK, and STAT protein families (Figure 3) [116].
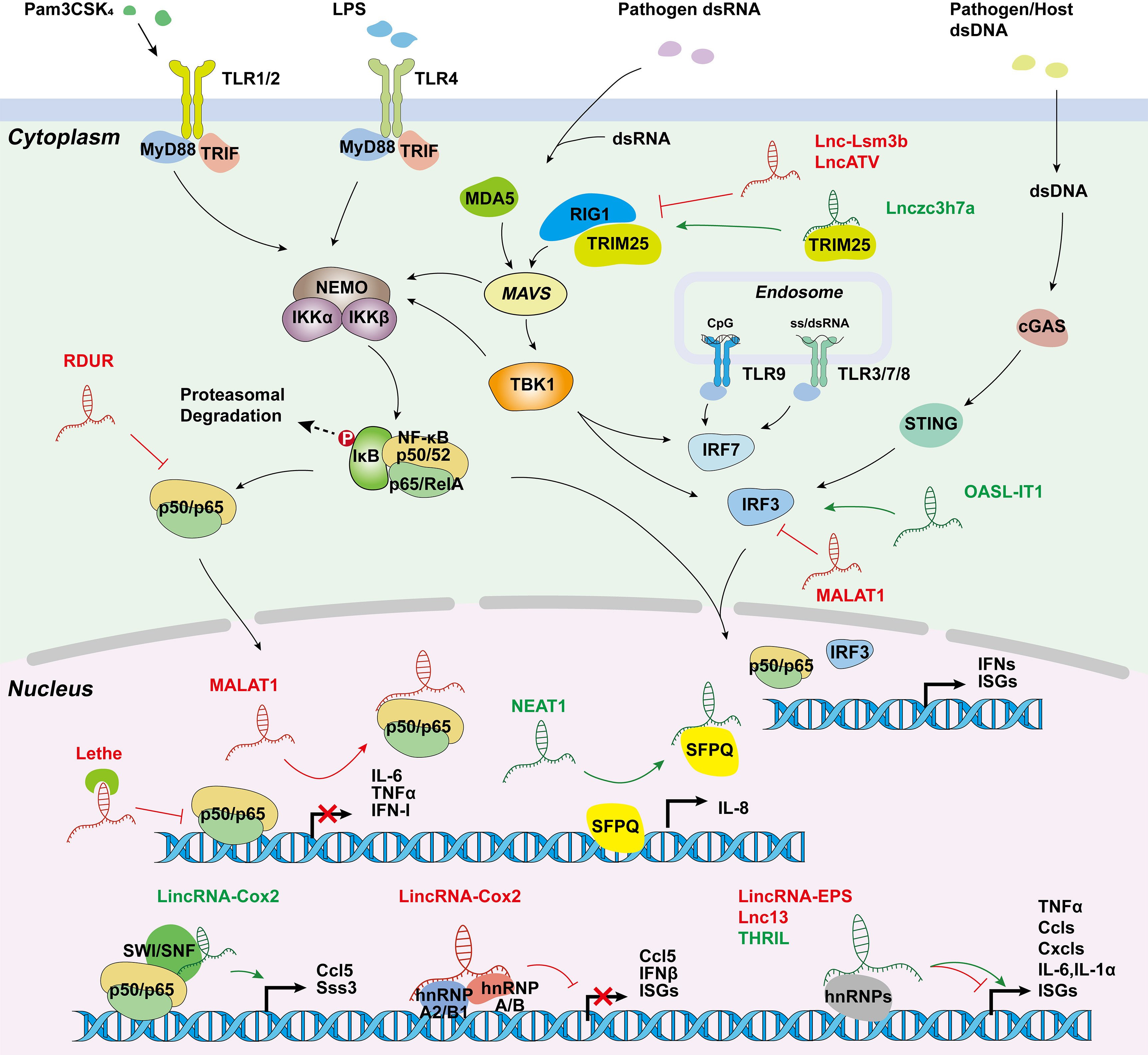
Figure 3. Long non-coding RNAs involved in innate immune responses. LincRNA-Cox2 activates or represses inflammatory genes through SWI/SNF-NF-κB complex or interactions with hnRNP-A/B and hnRNP-A2/B1; Lethe inhibits NF-κB locating target gene promoters; MALAT1 creates RNA-protein complex; NEAT1 initiates transcription by transferring SFPQ; and several other lncRNAs are involved in inflammation and virus-mediated innate immune response. hnRNP, heterogeneous nuclear ribonucleoprotein; SWI/SNF, switch/sucrose.
The NF-κB family contains seven distinct members, including NF-κB1 (p105 and p50), NF-κB2 (p100 and p52), RelA (p65), RelB and c-Rel, which can form a variety of dimers by interacting with each other [117]. TLR4 signaling leads to IKK complex activation and the subsequent phosphorylation and degradation of the inhibitor of NF-κB (IκB), which frees NF-κB that enters the nucleus and binds to a DNA motif, named the response elements (RE), initiating inflammatory gene transcription. Additionally, TLRs recruit IRAK and TRAF6, causing the activation of TGF-activated kinase 1 (TAK1) and MAPK family members (including p38, JNK, and ERK1/2), which mainly contributes to the expression of inflammatory mediators [118]. TAK1 can also activate the IKK complex leading to the release of NF-κB [119]. STAT3, a member of the STAT family, is another important transcriptional factor involved in immune responses, inflammation, and tumorigenesis. STAT3 activation requires the phosphorylation of Tyr705, which can be mediated by Janus kinases (JAKs, especially JAK2) [120] or activated by ROS accumulation [121], and then promotes the activation of inflammatory pathways, such as the NF-κB and IL6-GP130-JAK pathways [122].
Thus, NF-κB, MAPK, and STAT-JAK represent the primary signaling pathways and transcription factors that regulate inflammatory responses. As an active innate immune reaction to microenvironment challenge, inflammation is an essential protective response to maintain homeostasis. However, with the failure to clear noxious inflammatory materials or apoptotic inflammatory cells, inflammation may become chronic and lead to pathological lesion or chronic inflammatory diseases, such as cancers, arthritis, and cardiovascular diseases [123].
4.2.2. Long Non-coding RNAs Promote the Inflammatory Response
Studies have demonstrated the crucial functions of lncRNAs in promoting gene transcription, protein modification, and chromatin accessibility during the inflammatory response triggered by PRR activation. The first evidence that lncRNA expression can be induced in innate immune cells came from the studies in TLR4-activated cells. The expression of lincRNA-Cox2 (Table 2) is induced by more than 1000-fold in TLR4-stimulated CD11c+ bone-marrow-derived dendritic cells depending on the NF-κB pathway [124]. In addition, silencing of lincRNA-Cox2 led to the attenuated expression of 713 genes following Pam3CSK4 (a TLR1/2 agonist) stimulation. Mechanistically, in LPS-stimulated macrophages, the assembly of lincRNA-Cox2 into the switch/sucrose nonfermentable (SWI/SNF) complex is required for the incorporation of NF-κB subunits into the SWI/SNF complex, subsequently promoting histone H3 methylation and transactivation of late-primary inflammatory-response genes [125]. However, how lincRNA-Cox2 “guides” the recruitment of SWI/SNF complex to NF-κB responsive loci is unclear, but probably occurs through RNA-DNA duplex formation between lincRNA-Cox2 and the target gene loci. Despite its low basal expression level, the silencing of lincRNA-Cox2 in resting macrophages increased the expression of 787 genes, most of which are involved in the inflammatory response, such as Ccrl, Ccl5, Cx3cl1, and IFN-stimulated genes (e.g., Irf7, Oas1, and Isg15). The proposed mechanism of lincRNA-Cox2 for the transcriptional repression of the inflammatory genes is mediated by its interactions with hnRNP-A/B (encoded by Hnrnpab) and hnRNP-A2/B1 (encoded by Hnrnpa2b1), which inhibits inflammatory gene transcription in macrophages [11]. This research suggests that lincRNA-Cox2 has a dual role in regulating immune responses, depending on the cell context.
Table 2. Long non-coding RNAs act as modulators of inflammatory responses in innate immunity.
|
Model |
LncRNA |
Functional consequences |
Mechanism |
Reference |
|
Positive pattern |
LincRNA-Cox2 |
Transactivates inflammatory genes |
Incorporates NF-κB into the SWI/SNF complex |
[125] |
|
THRIL |
Promotes TLR2-mediated cytokines and chemokines expression |
Forms an RNA-protein complex with hnRNP |
[126] |
|
|
MALAT1 |
Promotes IL-1β, IL-6 and TNF-α expression |
Sponges miR-149 |
[127] |
|
|
LncRNA Sros1 |
Promotes IFN-γ-STAT1-mediated innate immunity |
Frees STAT1 mRNA from the RBP CAPRIN1 |
[128] |
|
|
LncRNA-155 |
Promotes IFN-β and ISGs production |
Inhibits PTP1B production |
[129] |
|
|
RDUR |
Upregulates IFNs and ISGs expression, alleviates inflammation |
Inactivates NF-κB |
[130] |
|
|
NEAT1 |
Promotes inflammasomes assembly, initiates antiviral gene IL-8 transcription |
Transfers the SFPQ from IL-8 promoter, maintains caspase-1 maturation |
||
|
Lnczc3h7a |
Activates TRIM25-mediated RIG-I antiviral response |
Forms trimeric complex with RIG-I and TRIM25 |
[133] |
|
|
OASL-IT1 |
Triggers IFN-β and ISGs expression, inhibits ZIKV infection |
Activates p38 MAPK, IRF3, and NF-κB |
[134] |
|
|
Negative pattern |
LincRNA-Cox2 |
Represses inflammatory response |
Interaction with hnRNP-A/B and hnRNP-A2/B1 |
[11] |
|
LincRNA-EPS |
Represses inflammatory genes expression |
Interaction with chromatin, hnRNPD or histone |
[10] |
|
|
Lnc13 |
Decreases inflammatory regulators expression |
Binds to hnRNPD p42 and Hdac1 on chromatin |
[135] |
|
|
Lethe |
Prevents proinflammatory cytokines production |
Prevents RelA-mediated transcription |
[136] |
|
|
MALAT1 |
Prevents proinflammatory cytokines and IFN-I production |
Binding to NF-κB, prevents IRF3 degradation |
[137]
|
|
|
Mirt2 |
Inhibits cytokine (e.g., IL-6, CXCL9) production |
Inactivates MAPK/NF-κB pathways |
[99] |
|
|
LncATV |
Inhibits IFNs and ISGs production |
Induces a mono-allelic mutation in the CARD of RIG-I |
[138] |
|
|
Lnc-Lsm3b |
Terminates type I IFNs production |
Limits RIG-I ubiquitination and phosphorylation |
[139] |
|
|
NRAV |
Inhibits transcription of ISGs |
Regulation on histone modification |
[140] |
THRIL, expressed in human tissues, is another lncRNA that participates in TNFα expression in response to stimulation with TLR2 ligand through forming an RNA–protein complex with hnRNP in THP1 macrophages [126]. The knockdown of THRIL decreased the expression of Pam-stimulated cytokines and chemokines including IL-8, TNFα, CCL1, CSF1, and CXCL10 among the more than 200 downregulated genes. Other proinflammatory lncRNAs, MALAT1 [127]and Sros1, [128] promote the expression of proinflammatory mediators via the derepression of MyD88/NF-κB as an miR-149 sponge in human lung injury inflammation and the activation of the STAT1 pathway by freeing Stat1 mRNA from the RBP CAPRIN1, respectively.
4.2.3. Long Non-Coding RNAs Inhibit the Inflammatory Response
Similar to the other non-coding RNAs, such as microRNAs that can be pro- or anti-inflammatory [141], lncRNAs have a dual effect on the regulation of innate immune responses. Transcriptome analysis demonstrates that lincRNA-EPS and lnc13, which are both localized in the nucleus, are two lncRNAs downregulated in macrophages after TLR activation, and repress the expression of inflammatory genes through association with chromatin at the regulatory sites of target genes, and binding to hnRNPD, respectively [10][135]. LPS-induced expression of chemokines (Ccl4, Ccl5, Cxcl2 and Cxcl10), cytokines (IL1α, IL6 and IL15), and antiviral ISGs (Ifit1, Ifi204, Oas2 and Rsad2/viperin) are potently declined in lincRNA-EPS expressing BMDMs. These results were confirmed by an enhanced inflammation response in lincRNA-EPS-deficient mice in vivo [10]. Similarly, the expression of master regulators of the inflammatory response, including TRAF2, MyD88, IL1RA, and STAT1, were potently enhanced in patients with Celiac disease associated with low expression of lnc13 in small intestine [135]. Another example of a lncRNA repressing inflammation is Lethe, a pseudogene lncRNA, primarily localized on the chromatin and highly induced by the proinflammatory cytokines IL-1β and TNF-α in mouse embryonic fibroblasts. Lethe functions as a negative feedback modulator of the NF-κB signaling pathway through interaction with NF-κB subunit RelA (p65), thus, preventing RelA from binding to the promoters of target genes, such as IL6 and IL8 [136]. In addition, Lethe-mediated blockage of RelA translocation into nucleus limits ROS production in macrophages, which may also contribute to the anti-inflammatory role of Lethe. The decreased Lethe expression and increased NADP oxidase gene expression observed in a mouse model of diabetic wound healing also support these findings [142]. MALAT1, another NF-κB repressor, restricts excessive inflammatory responses of LPS-activated macrophages by inhibiting NF-κB DNA binding activity [137]. Furthermore, as discussed above, Mirt2 inhibits cytokine (e.g., IL-6, CXCL9) production through the inactivation of MAPK/NF-κB pathways in macrophages [99]. Collectively, these studies highlight the crucial role of lncRNAs in the negative regulation of inflammatory response, which may provide potential strategies for the treatment of inflammatory diseases.
4.3. Long Non-Coding RNAs Function in Antiviral Innate Immune Response
In higher organisms, host antiviral innate immune response is triggered by the recognition of viral nucleic acids by PRRs, including TLR family members and the RLR family. After sensing viral invasion, a rapid induction of signaling cascades, such as type I and III IFN signaling [143], is initiated to coordinate innate immune cell behaviors with viral clearance. Although initial studies on the biological functions of lncRNAs in innate immunity primarily focused on host responses against bacteria, an increasing amount of research has demonstrated that thousands of lncRNAs are regulated by DNA or RNA virus infection. These lncRNAs may function to promote or inhibit viral replication and clearance by initiating or inactivating the induction of crucial viral sensors, IFN signaling, and the expression of direct viral clearance effectors.
4.3.1. Antiviral Signaling
Structural proteins of viral envelope and capsid are major PAMPs that are recognized by serval TLRs present in the cell membrane, such as TLR2 and TLR4, while viral RNA or DNA, when released into the cytoplasm of infected host cells, are recognized by endosomal TLRs: for example, TLR3 senses double-stranded RNA [144], TLR7/8 recognize degradation products of single-stranded RNA (ssRNA) [145], and TLR9 is specific to DNA with unmethylated CpG [146].
The RLRs are a family that detect cytosolic viral RNAs and are essential for the initiation of the innate immune response against RNA viruses. RLR sensors include three members: RIG-I, melanoma differentiation-associated gene 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2), which are similarly organized and share a central DExD/H box helicase domain [147]. RIG-I and MDA5 have two N-terminal caspase activation and recruitment domains (CARDs) that are responsible for the interaction between activated RIG-I or MDA5 and the adaptor protein mitochondrial antiviral signaling (MAVS), which mediates the activation of NF-κB, IRF3, IRF7, and ATF2 in response to viral infection [143]. In addition, cyclic GMP-AMP synthase (cGAS) is another important sensor, which recognizes cytosolic double-stranded DNA and initiates the secretion of type I IFN and other inflammatory cytokines [148]. Moreover, the cytosolic viral DNA induces NLRP3 inflammasome complex formation through ROS, or AIM2 associated ASC adapter and pro-caspase-1, which converts pro-caspase-1 into its active form cleaving pro-IL-1β and pro-IL-18 into mature forms [149][150].
The common outcome of these signaling pathways is transcription of the inflammatory genes initiated by NF-κB or ATF2, and the transcription of important antiviral genes (such as type I IFN, IFN-α, and IFN-β) initiated by IRF3 or IRF7, responsible for viral clearance.
4.3.2. Long Non-coding RNAs Promote Antiviral Innate Immune Response
Like the inflammatory signaling pathways, antiviral signaling is also strictly regulated by lncRNAs. Influenza A virus (IAV) is a common pathogen that causes respiratory tract infections and constitutes a major threat to human health. Several lncRNAs, such as lncRNA-155, RIG-I-dependent IAV-upregulated non-coding RNA (RDUR), and NEAT1, are upregulated in innate immune cells upon IAV infection. TLR3 and RIG-1 induced upregulation of lncRNA-155, both in vitro and in vivo (mouse model), promotes the production of IFN-β and several vital IFN-stimulated genes (ISGs), such as Mx1, Isg15, and Oas3, by inhibiting protein tyrosine phosphatase 1B (PTP1B) expression [129]. NF-κB-mediated increase in RDUR expression plays a similar role in promoting antiviral molecule expression (e.g., IFNs and ISGs) in vitro and in vivo; meanwhile, it inactivates NF-κB to prevent an excessive inflammatory response through a negative feedback mechanism [130]. NEAT1 has been found to be associated with cytokine IL-8 expression in response to dsRNA-mediated TLR3 activation or viral infections, such as IAV and Herpes simplex virus type (HSV-1) infection. It initiates IL-8 transcription by binding to and transferring the repressor splicing factor proline/glutamine-rich (SFPQ) from the IL-8 promoter to the nuclear paraspeckle bodies [132]]. NEAT1 also promotes AIM2, NLRP3, or NLRP4 inflammasome assembly and maintains a mature caspase-1 for IL-1β production and pyroptosis [131].
Moreover, RIG-1 signaling is also enhanced by Lnczc3h7a, which forms one stable trimeric complex by binding to TRIM25 in macrophages upon the infection of RNA or DNA viruses and IFN-β stimulation. This complex acts as a scaffold to promote and stabilize the interaction between TRIM25 and the activated RIG-I, and then strengthens TRIM25-mediated K63-linked ubiquitination of RIG-I [133]. In epithelial A549 cells, researchers found that lncRNA OASL-IT1 promotes the phosphorylation of p38 MAPK, IRF3, and NF-κB p65, leading to the expression of IFN-β and two classic ISGs (MX1 and IFITM1) in a positive feedback manner during Zika virus (ZIKV) infection [134].
4.3.3. Long Non-Coding RNAs Inhibit Antiviral Innate Immune Response
Most recently, an array of novel lncRNAs were found to regulate the virus-related innate immune response in a negative manner. Cytoplasmic lncATV is highly expressed in human monocytes, hepatoma cells, and erythroleukemia cells, and is upregulated upon type I/III IFN stimulation and infection of viruses, such as hepatitis C virus, Sendai virus, Newcastle disease virus, and ZIKV. LncATV potently inhibits RIG-I antiviral signaling and the IFN pathway, probably due to its association with RIG-I [138]. Similar to lncATV, lncRNA lnc-Lsm3b, which is upregulated upon virus infection, blocks RIG-1 activation through binding to the CARD and helicase domain of RIG-1, limiting RIG-I ubiquitination and phosphorylation, and reducing virus-induced IFN-β and NF-κB promoter activity. This was also confirmed in lnc-Lsm3b-deficient mice [139].
Different from lncATV and lnc-Lsm3b, MALAT1 is downregulated in macrophages infected with viruses. A reduction in MALAT1 expression is required for caspase-3-mediated TDP43 to TDP35 activation in nucleus, which prevents IRF3 from proteasomal degradation and promotes type I IFN production. Additionally, MALAT1-deficient mice show enhanced antiviral response after VSV or HSV-1 infection [151]. LncRNA NRAV inhibits the initial transcription of multiple critical ISGs, including MxA and IFITM3, by regulating the histone modification of these genes, and causes IAV replication and virulence in human cells and transgenic mice expressing human NRAV. It is demonstrated that the formation of the spatial structure of NRAV stem loops, except one small arm (nt 618-872), is necessary for NRAV biological function during virus infection [140].
5. Innate Immune Long Non-Coding RNAs in Non-Infectious Diseases
In addition to infectious diseases, inappropriately engaged or dysregulated inflammatory process may disturb tissue homeostasis and cause extensive autoimmune diseases, such as chronic auto-inflammatory diseases, atopic dermatitis, cardiovascular diseases, obesity, and type 2 diabetes [152]. Genome-wide association studies (GWAS) showed that more than 90% of disease-related SNPs occur in non-coding regions [153] and approximately 10% of SNPs associated with immune and autoimmune disorders are found in lncRNA loci [154]. Moreover, altered expression of lncRNAs has been found in several inflammatory diseases; thus, with future prospective studies, lncRNAs could represent a new therapeutic target for this type of disease.
5.1. Hematological Diseases
Prolonged inflammation caused by dysregulated innate immune cell survival leads to many human inflammatory and hematological diseases [155][156]; thus, the lifespan of innate immune cells must be strictly controlled. Hypereosinophilic syndrome (HES) is a kind of disorder characterized by eosinophilia associated with increased responsiveness to IL-5. A lncRNA Morrbid, elevated in some HES patients, plays a critical role in the development of HES by inhibiting the apoptosis of eosinophils [157]. Morrbid represses the transcription of its neighboring pro-apoptotic gene Bcl2l11 by promoting the enrichment of PRC2 and subsequent deposition of repressive H3K27me3 at the bivalent promoter of Bcl2l11 in short-lived myeloid cells, including neutrophils, eosinophils, and classical monocytes, in response to pro-survival cytokines, like IL-5. Moreover, impaired hematopoietic differentiation in leukemia may also be a result of dysregulated lncRNAs, for example, HOTAIRM1 and NEAT1. As mentioned above, the lack of HOTAIRM1 suppresses the activation of HoxA1 and HoxA4, leading to granulocytic differentiation blockade in NB4 acute promyelocytic leukemia cell line [103]. Promyelocytic leukemia with retinoic acid receptor alpha (PML-RARα)-fusion-mediated NEAT1 transcriptional repression might impair the myelopoiesis of acute promyelocytic leukemia cells [158]. Although these studies suggest a pathological function of lncRNAs in hematological diseases, most are based on in vitro experiments and further experimentation in vivo will be required.
5.2. Rheumatoid Arthritis
Rheumatoid arthritis (RA) is one prevalent chronic inflammatory disorder, characterized by persistent synovitis in the joints [159]. Although the precise etiology of RA is still unclear, both genetic and environmental factors have been identified as important contributors to RA development. In recent years, several studies have revealed the functional role of lncRNAs in peripheral blood mononuclear cells (PBMCs) or fibroblast-like synoviocytes (FLSs) in RA pathology. Hotari is one of the firstly reported lncRNAs and notably expressed in the PBMCs and serum exosomes of RA patients[160]. The upregulated Hotari in RA exosomes may induce the migration of activated macrophages to the joints. Moreover, Hotair activates MMP-2 and MMP-13 in osteoclasts and synoviocytes, which may lead to proteinase-mediated dissolution of articular cartilage matrix and subchondral bone resorption during RA pathogenesis. In contrast, lncRNA CASC2 is downregulated in the plasma of RA patients [161]. Through decreasing IL-17 expression, CASC2 could potently promote the apoptosis of FLSs that contribute to RA development by producing cytokines and proteases [161]. However, due to the complicated pathogenic mechanism of autoimmune disease, there is extensive lncRNA regulatory potential that has yet to be discovered in RA development.
5.3. Cardiovascular Diseases
Cardiovascular disease is a major cause of mortality and morbidity in patients with chronic inflammatory disorders, such as the RA described above. The cytokines and chemokines produced by innate immune cells during the chronic inflammatory response not only act as biomarkers but also directly contribute to the pathogenesis of cardiovascular diseases, where macrophages play a crucial pathological role [162]. Macrophage accumulation within vascular intima leads to persistent local inflammatory responses, causing atherosclerosis [163]. An array of lncRNAs have been reported in the tight regulation of macrophage phenotypes during the progression of atherosclerosis, such as lncRNA RAPIA [164], MAARS [165], MIAT [166], PELATON [167], Mirt2 [99], by controlling the inflammatory process, lipid homeostasis, and cell cycle. LncRNAs also play an important role in maintaining the hemostasis of endothelial cells that are nonprofessional innate immune cells, during the development of cardiovascular diseases, such as lncRNA SRA [168], NEXN-AS1/NEXN [169], lncRNA-CCL2 [170]. However, considering that cardiovascular disease is the leading cause of death worldwide, the current understanding of lncRNAs contributing to this pathogenic process is still insufficient.
5.4. Intestinal Diseases
Inflammatory bowel disease (IBD) represents a group of intestinal disorders characterized by prolonged inflammation of the digestive tract and systemic release of the luminal microbiota due to an imbalance between the intestinal immune system and microbiota. A growing body of evidence has demonstrated that innate immune lncRNAs are involved in the pathogenesis of IBD, including ulcerative colitis and Crohn’s disease. In accordance with its proinflammatory role, genetic variants decreasing Lnc-ROCKI expression in human monocytes reduce the risk of IBD and other inflammatory diseases (such as atherosclerosis) [171]. However, most research into lncRNA functions in IBD focuses on nonprofessional innate immune cells, such as intestinal epithelial cells (IECs). A lncRNA HIF1A-AS2 is upregulated in mice with ulcerative colitis induced by Flagellin and inhibits cytokine expression in IEC-like cells as well as alleviating colonic inflammation in vivo [172]. The suppression of ANRIL alleviated LPS-induced injury in fetal human cells and inhibited the development of ulcerative colitis through the TLR4/MyD88/NF-κB pathway by negatively regulating miR-323b-5p [173].
Celiac disease (CeD), a chronic and innate immune-mediated intestinal disorder, is closely associated with non-coding regions of human genome[174][175]. Studies have revealed that lnc13 levels are decreased in small intestine of CeD patients, repressing the expression of inflammatory genes (e.g., Stat1, Stat3, Traf2, Myd88, Ccl12, Il1ra) in macrophages, indicating a potential downregulation role for lnc13 in the pathogenesis of CeD [135]. Other lncRNAs, such as Neat1 and TUG1 also participate in DeD disease by association with STAT3 [176].
5.5. Diabetes Mellitus
Diabetes mellitus (DM) is a metabolic disorder characterized by chronic low-grade inflammation in the pancreatic islets and impaired insulin secretory capacity. Over the last decade, human transcriptome analyses have shown that lncRNAs are dynamically regulated and abnormally expressed in patients with DM, such as MALAT1, uc.48+, E330013P06, Hotair, Miat, and GAS5, and might be potential diagnostic biomarkers for DM [177][178][179][180]. MALAT1 was found to improve DM-induced retinal endothelial cell dysfunction and microvascular abnormalities by activating the p38/MAPK signaling pathway [177]. A lncRNA uc.48+ are responsible for the development of type 2 diabetes (T2DM) by inducing P2X7P-mediated immune, ERK1/2-mediated proinflammatory response, and ROS formation in the RAW264.7 macrophage [178]. Moreover, E330013P06 contributes to the increased susceptibility of T2DM through the enhancement of the inflammatory response and macrophage-derived foam cell formation [179]. However, the functional role of lncRNAs in innate immune cells during DM development is still being established.
6. Concluding Remarks and Future Perspectives
Tremendous progress made in recent years has provided clear evidence that lncRNAs play an important role in the regulation of innate immunity. Although we have focused on exploring the functional roles and mechanisms of these RNAs, a large void in our understanding of how these lncRNAs function at the molecular level in the context of innate immune response remains. The innate immune response initiates with the recognition of pathogens through PRRs by innate immune cells accompanied by the development of inflammatory response. A large amount of inflammation-related signaling pathways and molecules, including classical TLRs, NF-κB, cytokines, and chemokines, are involved in this progress. Studies on lncRNA biology and functions greatly expanded our knowledge of how genes associated with inflammation and innate immune response are regulated. Various lncRNAs participate in the development and differentiation of innate immune cells, key molecule transcription, signaling transduction and disease development through versatile mechanisms, in either a positive or negative pattern. Nevertheless, given its complexity, what determines the net effect that the incorporated network between regulatory lncRNAs and inflammatory signaling pathways has on the pathophysiological fate of the immune system remains to be fully understood.
In addition, lncRNAs regulate transcriptional programs through diverse mechanisms including interactions with chromatin, DNA, RNA, and proteins either independent of or dependent on specific sequences. Less sequence conservation is not necessarily equivalent to fewer biological functions. However, we still have a poor understanding of how selective pressures act on lncRNAs at the sequence and structural levels, raising questions about what determines the evolution and function of lncRNAs. For instance, what are the key motifs or signatures for lncRNAs that have tissue- or cell-specific functions depending on their sequences? What is the role of alternative splicing for those lncRNAs that function through their transcript and processing independent of sequences? Do lncRNAs that share similar functions have similar and specific features? Additionally, a small number of lncRNAs are found to encode small peptides, which has not been investigated in detail. Finally, since the number of described lncRNAs is rapidly increasing due to the wide-range application of high-throughput sequencing technologies, an illustration of the exact molecular mechanisms underlying the biological functions of lncRNAs will be the greatest challenge in lncRNA studies.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22179535
References
- Akiko Iwasaki; Ruslan Medzhitov; Control of adaptive immunity by the innate immune system. Nature Immunology 2015, 16, 343-353, 10.1038/ni.3123.
- Osamu Takeuchi; Shizuo Akira; Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805-820, 10.1016/j.cell.2010.01.022.
- Susan Carpenter; Long noncoding RNA: Novel links between gene expression and innate immunity. Virus Research 2016, 212, 137-145, 10.1016/j.virusres.2015.08.019.
- Yiwen Fang; Melissa Fullwood; Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genomics, Proteomics & Bioinformatics 2016, 14, 42-54, 10.1016/j.gpb.2015.09.006.
- Troyee Das; Aritra Deb; Sibun Parida; Sudip Mondal; Sunirmal Khatua; Zhumur Ghosh; LncRBase V.2: an updated resource for multispecies lncRNAs and ClinicLSNP hosting genetic variants in lncRNAs for cancer patients. RNA Biology 2020, 18, 1136-1151, 10.1080/15476286.2020.1833529.
- Paulo P. Amaral; Tommaso Leonardi; Namshik Han; Emmanuelle Vire; Dennis K. Gascoigne; Raúl Arias-Carrasco; Magdalena Büscher; Luca Pandolfini; Anda Zhang; Stefano Pluchino; et al. Genomic positional conservation identifies topological anchor point RNAs linked to developmental loci. Genome Biology 2018, 19, 1-21, 10.1186/s13059-018-1405-5.
- Thomas Derrien; Rory Johnson; Giovanni Bussotti; Andrea Tanzer; Sarah Djebali; Hagen Tilgner; Gregory Guernec; David Martin; Angelika Merkel; David G. Knowles; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Research 2012, 22, 1775-1789, 10.1101/gr.132159.111.
- Y Grace Chen; Ansuman Satpathy; Howard Y Chang; Gene regulation in the immune system by long noncoding RNAs. Nature Immunology 2017, 18, 962-972, 10.1038/ni.3771.
- Maite Huarte; Mitchell Guttman; David Feldser; Manuel Garber; Magdalena Koziol; Daniela Kenzelmann-Broz; Ahmad M. Khalil; Or Zuk; Ido Amit; Michal Rabani; et al. A Large Intergenic Noncoding RNA Induced by p53 Mediates Global Gene Repression in the p53 Response. Cell 2010, 142, 409-419, 10.1016/j.cell.2010.06.040.
- Maninjay K. Atianand; Wenqian Hu; Ansuman Satpathy; Ying Shen; Emiliano Ricci; Juan R. Alvarez-Dominguez; Ankit Bhatta; Stefan Schattgen; Jason D. McGowan; Juliana Blin; et al. A Long Noncoding RNA lincRNA-EPS Acts as a Transcriptional Brake to Restrain Inflammation. Cell 2016, 165, 1672-1685, 10.1016/j.cell.2016.05.075.
- Susan Carpenter; Daniel Aiello; Maninjay K. Atianand; Emiliano Ricci; Pallavi Gandhi; Lisa L. Hall; Meg Byron; Brian Monks; Meabh Henry-Bezy; Jeanne B. Lawrence; et al. A Long Noncoding RNA Mediates Both Activation and Repression of Immune Response Genes. Science 2013, 341, 789-792, 10.1126/science.1240925.
- The ENCODE Project Consortium; An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57-74, 10.1038/nature11247.
- Sarah Djebali; Carrie A. Davis; Angelika Merkel; Alex Dobin; Timo Lassmann; Ali Mortazavi; Andrea Tanzer; Julien Lagarde; Wei Lin; Felix Schlesinger; et al. Landscape of transcription in human cells. Nature 2012, 489, 101-108, 10.1038/nature11233.
- Jennifer Harrow; Adam Frankish; Jose M Gonzalez; Electra Tapanari; Mark Diekhans; Felix Kokocinski; Bronwen Aken; Daniel Barrell; Amonida Zadissa; Stephen Searle; et al. GENCODE: The reference human genome annotation for The ENCODE Project. Genome Research 2012, 22, 1760-1774, 10.1101/gr.135350.111.
- John L. Rinn; Howard Y. Chang; Genome Regulation by Long Noncoding RNAs. Annual Review of Biochemistry 2012, 81, 145-166, 10.1146/annurev-biochem-051410-092902.
- Mattick, J.S.; Rinn, J.L.; Discovery and annotation of long noncoding RNAs. Nat. Struct. Mol. Biol 2015, 22, 5–7, Mattick, J. S., & Rinn, J. L. (2015). Discovery and annotation of long noncoding RNAs. Nature Structural & Molecular Biology, 22(1), 5–7. doi:10.1038/nsmb.2942 .
- Gioacchino Natoli; Jean-Christophe Andrau; Noncoding Transcription at Enhancers: General Principles and Functional Models. Annual Review of Genetics 2012, 46, 1-19, 10.1146/annurev-genet-110711-155459.
- Andreas Werner; Mark Carlile; Daniel Swan; What do natural antisense transcripts regulate?. RNA Biology 2009, 6, 43-48, 10.4161/rna.6.1.7568.
- Michael Clark; Rebecca Johnston; Mario Inostroza-Ponta; Archa Fox; Ellen Fortini; Pablo Moscato; Marcel E. Dinger; John Mattick; Genome-wide analysis of long noncoding RNA stability. Genome Research 2012, 22, 885-898, 10.1101/gr.131037.111.
- Jessica A. Brown; Max L. Valenstein; Therese A. Yario; Kazimierz T. Tycowski; Joan A. Steitz; Formation of triple-helical structures by the 3'-end sequences of MALAT1 and MEN noncoding RNAs. Proceedings of the National Academy of Sciences 2012, 109, 19202-19207, 10.1073/pnas.1217338109.
- Jeremy E. Wilusz; Courtney K. JnBaptiste; Laura Y. Lu; Claus-D. Kuhn; Leemor Joshua-Tor; Phillip A. Sharp; A triple helix stabilizes the 3' ends of long noncoding RNAs that lack poly(A) tails. Genes & Development 2012, 26, 2392-2407, 10.1101/gad.204438.112.
- Margarita Schlackow; Takayuki Nojima; Tomás Gomes; Ashish Dhir; Maria Carmo-Fonseca; Nick J. Proudfoot; Distinctive Patterns of Transcription and RNA Processing for Human lincRNAs. Molecular Cell 2016, 65, 25-38, 10.1016/j.molcel.2016.11.029.
- Yafei Yin; J. Yuyang Lu; Xuechun Zhang; Wen Shao; Yanhui Xu; Pan Li; Yantao Hong; Li Cui; Ge Shan; Bin Tian; et al. U1 snRNP regulates chromatin retention of noncoding RNAs. Nature 2020, 580, 147-150, 10.1038/s41586-020-2105-3.
- Hadas Hezroni; David Koppstein; Matthew G. Schwartz; Alexandra Avrutin; David P. Bartel; Igor Ulitsky; Principles of Long Noncoding RNA Evolution Derived from Direct Comparison of Transcriptomes in 17 Species. Cell Reports 2015, 11, 1110-1122, 10.1016/j.celrep.2015.04.023.
- Serge Saxonov; Paul Berg; Douglas L. Brutlag; A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proceedings of the National Academy of Sciences 2006, 103, 1412-1417, 10.1073/pnas.0510310103.
- Alexander E. Vinogradov; Dualism of gene GC content and CpG pattern in regard to expression in the human genome: magnitude versus breadth. Trends in Genetics 2005, 21, 639-643, 10.1016/j.tig.2005.09.002.
- Simon Cawley; Stefan Bekiranov; Huck H Ng; Philipp Kapranov; Edward A Sekinger; Dione Kampa; Antonio Piccolboni; Victor Sementchenko; Jill Cheng; Alan J Williams; et al. Unbiased Mapping of Transcription Factor Binding Sites along Human Chromosomes 21 and 22 Points to Widespread Regulation of Noncoding RNAs. Cell 2004, 116, 499-509, 10.1016/s0092-8674(04)00127-8.
- Masahiro Uesaka; Osamu Nishimura; Yasuhiro Go; Kinichi Nakashima; Kiyokazu Agata; Takuya Imamura; Bidirectional promoters are the major source of gene activation-associated non-coding RNAs in mammals. BMC Genomics 2014, 15, 35-35, 10.1186/1471-2164-15-35.
- Chiara Braconi; T Kogure; Nicola Valeri; N Huang; G Nuovo; S Costinean; Massimo Negrini; E Miotto; C M Croce; T Patel; et al. microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene 2011, 30, 4750-4756, 10.1038/onc.2011.193.
- Ira W. Deveson; Marion E. Brunck; James Blackburn; Elizabeth Tseng; Ting Hon; Tyson A. Clark; Michael B. Clark; Joanna Crawford; Marcel E. Dinger; Lars K. Nielsen; et al. Universal Alternative Splicing of Noncoding Exons. Cell Systems 2018, 6, 245-255.e5, 10.1016/j.cels.2017.12.005.
- Binyamin Zuckerman; Igor Ulitsky; Predictive models of subcellular localization of long RNAs. RNA 2019, 25, 557-572, 10.1261/rna.068288.118.
- Je-Hyun Yoon; Kotb Abdelmohsen; Myriam Gorospe; Functional interactions among microRNAs and long noncoding RNAs. Seminars in Cell & Developmental Biology 2014, 34, 9-14, 10.1016/j.semcdb.2014.05.015.
- Alberto R. Kornblihtt; Ignacio E. Schor; Mariano Allo; Gwendal Dujardin; Ezequiel Petrillo; Manuel J. Muñoz; Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nature Reviews Molecular Cell Biology 2013, 14, 153-165, 10.1038/nrm3525.
- Xiang-Dong Fu; Manuel Ares; Context-dependent control of alternative splicing by RNA-binding proteins. Nature Reviews Microbiology 2014, 15, 689-701, 10.1038/nrg3778.
- Anamaria Necsulea; Magali Soumillon; Maria Warnefors; Angélica Liechti; Tasman Daish; Ulrich Zeller; Julie C. Baker; Frank Grützner; Henrik Kaessmann; The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature 2014, 505, 635-640, 10.1038/nature12943.
- Jin-Wu Nam; David P. Bartel; Long noncoding RNAs in C. elegans. Genome Research 2012, 22, 2529-2540, 10.1101/gr.140475.112.
- Jarroux, J.; Morillon, A.; Pinskaya, M. History, Discovery, and Classification of lncRNAs; Springer: Singapore, 2017; pp. pp. 1–46.
- Anne Nitsche; Dominic Rose; Mario Fasold; Kristin Reiche; Peter F. Stadler; Comparison of splice sites reveals that long noncoding RNAs are evolutionarily well conserved. RNA 2015, 21, 801-812, 10.1261/rna.046342.114.
- Xun Zhang; Kimberley Rice; Yingying Wang; Wendy Chen; Ying Zhong; Yuki Nakayama; Yunli Zhou; Anne Klibanski; Maternally Expressed Gene 3 (MEG3) Noncoding Ribonucleic Acid: Isoform Structure, Expression, and Functions. Endocrinology 2009, 151, 939-947, 10.1210/en.2009-0657.
- Jian-Rong Yang; Jianzhi Zhang; Human Long Noncoding RNAs Are Substantially Less Folded than Messenger RNAs. Molecular Biology and Evolution 2015, 32, 970-977, 10.1093/molbev/msu402.
- Pornchai Kaewsapsak; David Michael Shechner; William Mallard; John L Rinn; Alice Y Ting; Live-cell mapping of organelle-associated RNAs via proximity biotinylation combined with protein-RNA crosslinking. eLife 2017, 6, e29224, 10.7554/elife.29224.
- John Rinn; Mitchell Guttman; RNA and dynamic nuclear organization. Science 2014, 345, 1240-1241, 10.1126/science.1252966.
- Jesse M. Engreitz; Noah Ollikainen; Mitchell Guttman; Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nature Reviews Molecular Cell Biology 2016, 17, 756-770, 10.1038/nrm.2016.126.
- Yoav Lubelsky; Igor Ulitsky; Sequences enriched in Alu repeats drive nuclear localization of long RNAs in human cells. Nature Cell Biology 2018, 555, 107-111, 10.1038/nature25757.
- Hongjae Sunwoo; David Colognori; John Froberg; Yesu Jeon; Jeannie T. Lee; Repeat E anchors Xist RNA to the inactive X chromosomal compartment through CDKN1A-interacting protein (CIZ1). Proceedings of the National Academy of Sciences 2017, 114, 10654-10659, 10.1073/pnas.1711206114.
- Stefan Bresson; Olga V. Hunter; Allyson C. Hunter; Nicholas K. Conrad; Canonical Poly(A) Polymerase Activity Promotes the Decay of a Wide Variety of Mammalian Nuclear RNAs. PLOS Genetics 2015, 11, e1005610, 10.1371/journal.pgen.1005610.
- Jeffrey M. Levsky; Robert H. Singer; Fluorescence in situ hybridization: past, present and future. Journal of Cell Science 2003, 116, 2833-2838, 10.1242/jcs.00633.
- Arjun Raj; Patrick Van Den Bogaard; Scott A Rifkin; Alexander van Oudenaarden; Sanjay Tyagi; Imaging individual mRNA molecules using multiple singly labeled probes. Nature Methods 2008, 5, 877-879, 10.1038/nmeth.1253.
- Hyun-Woo Rhee; Peng Zou; Namrata D. Udeshi; Jeffrey D. Martell; Vamsi K. Mootha; Steven A. Carr; Alice Y. Ting; Proteomic Mapping of Mitochondria in Living Cells via Spatially Restricted Enzymatic Tagging. Science 2013, 339, 1328-1331, 10.1126/science.1230593.
- Stephanie Shih-Min Lam; Jeffrey Daniel Martell; Kimberli J. Kamer; Thomas J. Deerinck; Mark H. Ellisman; Vamsi K. Mootha; Alice Y. Ting; Directed evolution of APEX2 for electron microscopy and proximity labeling. Nature Methods 2014, 12, 51-54, 10.1038/nmeth.3179.
- Jiahui Wu; Sara Zaccara; Deepak Khuperkar; Hyaeyeong Kim; Marvin E. Tanenbaum; Samie R. Jaffrey; Live imaging of mRNA using RNA-stabilized fluorogenic proteins. Nature Methods 2019, 16, 862-865, 10.1038/s41592-019-0531-7.
- Furqan M. Fazal; Shuo Han; Kevin R. Parker; Pornchai Kaewsapsak; Jin Xu; Alistair N. Boettiger; Howard Y. Chang; Alice Y. Ting; Atlas of Subcellular RNA Localization Revealed by APEX-Seq. Cell 2019, 178, 473-490.e26, 10.1016/j.cell.2019.05.027.
- Walter Mowel; Jonathan J. Kotzin; Sam McCright; Vanessa D. Neal; Jorge Henao-Mejia; Control of Immune Cell Homeostasis and Function by lncRNAs. Trends in Immunology 2017, 39, 55-69, 10.1016/j.it.2017.08.009.
- Kevin C. Wang; Howard Y. Chang; Molecular Mechanisms of Long Noncoding RNAs. Molecular Cell 2011, 43, 904-914, 10.1016/j.molcel.2011.08.018.
- Tianwen Li; Xiaoyan Mo; Liyun Fu; Bingxiu Xiao; Junming Guo; Molecular mechanisms of long noncoding RNAs on gastric cancer. Oncotarget 2016, 7, 8601-8612, 10.18632/oncotarget.6926.
- Anna Postepska-Igielska; Alena Giwojna; Lital Gasri-Plotnitsky; Nina Schmitt; Annabelle Dold; Doron Ginsberg; Ingrid Grummt; LncRNA Khps1 Regulates Expression of the Proto-oncogene SPHK1 via Triplex-Mediated Changes in Chromatin Structure. Molecular Cell 2015, 60, 626-636, 10.1016/j.molcel.2015.10.001.
- Jennifer E. Phillips; Victor G. Corces; CTCF: Master Weaver of the Genome. Cell 2009, 137, 1194-1211, 10.1016/j.cell.2009.06.001.
- Jian-Feng Xiang; Qing-Fei Yin; Tian Chen; Yang Zhang; Xiao-Ou Zhang; Zheng Wu; Shaofeng Zhang; Hai-Bin Wang; Junhui Ge; Xuhua Lu; et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Research 2014, 24, 513-531, 10.1038/cr.2014.35.
- Anke Sparmann; Maarten Van Lohuizen; Polycomb silencers control cell fate, development and cancer. Nature Reviews Cancer 2006, 6, 846-856, 10.1038/nrc1991.
- Rajnish A. Gupta; Nilay Shah; Kevin C. Wang; Jeewon Kim; Hugo M. Horlings; David J. Wong; Miao-Chih Tsai; Tiffany Hung; Pedram Argani; John Rinn; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071-1076, 10.1038/nature08975.
- Annette M Krecic; Maurice S Swanson; hnRNP complexes: composition, structure, and function. Current Opinion in Cell Biology 1999, 11, 363-371, 10.1016/s0955-0674(99)80051-9.
- Nadya Dimitrova; Jesse R. Zamudio; Robyn M. Jong; Dylan Soukup; Rebecca Resnick; Kavitha Sarma; Amanda J. Ward; Arjun Raj; Jeannie T. Lee; Phillip A. Sharp; et al. LincRNA-p21 Activates p21 In cis to Promote Polycomb Target Gene Expression and to Enforce the G1/S Checkpoint. Molecular Cell 2014, 54, 777-790, 10.1016/j.molcel.2014.04.025.
- Xiaodong Song; Pan Xu; Chao Meng; Chenguang Song; Timothy S. Blackwell; Rongrong Li; Hongbo Li; Jinjin Zhang; Changjun Lv; lncITPF Promotes Pulmonary Fibrosis by Targeting hnRNP-L Depending on Its Host Gene ITGBL1. Molecular Therapy 2018, 27, 380-393, 10.1016/j.ymthe.2018.08.026.
- Ying Shen; Yuandong Feng; Fangmei Li; Yachun Jia; Yue Peng; Wanhong Zhao; Jinsong Hu; Aili He; lncRNA ST3GAL6‑AS1 promotes invasion by inhibiting hnRNPA2B1‑mediated ST3GAL6 expression in multiple myeloma. International Journal of Oncology 2021, 58, 1-1, 10.3892/ijo.2021.5185.
- Margaret S. Ebert; Phillip A. Sharp; Emerging Roles for Natural MicroRNA Sponges. Current Biology 2010, 20, R858-R861, 10.1016/j.cub.2010.08.052.
- Leonardo Salmena; Laura Poliseno; Yvonne Tay; Lev Kats; Pier Paolo Pandolfi; A ceRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language?. Cell 2011, 146, 353-358, 10.1016/j.cell.2011.07.014.
- Ding-Wen Cao; Man-Man Liu; Rui Duan; Yi-Fu Tao; Jun-Shan Zhou; Wei-Rong Fang; Jun-Rong Zhu; Li Niu; Jian-Guo Sun; The lncRNA Malat1 functions as a ceRNA to contribute to berberine-mediated inhibition of HMGB1 by sponging miR-181c-5p in poststroke inflammation. Acta Pharmacologica Sinica 2019, 41, 22-33, 10.1038/s41401-019-0284-y.
- Hu Chen; Xiaoyun Wang; Xuetao Yan; Xiaoli Cheng; Xianghu He; Wenzhong Zheng; RETRACTED: LncRNA MALAT1 regulates sepsis-induced cardiac inflammation and dysfunction via interaction with miR-125b and p38 MAPK/NFκB. International Immunopharmacology 2018, 55, 69-76, 10.1016/j.intimp.2017.11.038.
- Lingling Dai; Guojun Zhang; Zhe Cheng; Xi Wang; Liuqun Jia; Xiaogang Jing; Huan Wang; Rui Zhang; Meng Liu; Tianci Jiang; et al. Knockdown of LncRNA MALAT1 contributes to the suppression of inflammatory responses by up-regulating miR-146a in LPS-induced acute lung injury. Connective Tissue Research 2018, 59, 581-592, 10.1080/03008207.2018.1439480.
- Heng-Jun Zhou; Li-Qing Wang; Duan-Bu Wang; Jian-Bo Yu; Yu Zhu; Qing-Sheng Xu; Xiu-Jue Zheng; Ren-Ya Zhan; Long noncoding RNA MALAT1 contributes to inflammatory response of microglia following spinal cord injury via the modulation of a miR-199b/IKKβ/NF-κB signaling pathway. American Journal of Physiology-Cell Physiology 2018, 315, C52-C61, 10.1152/ajpcell.00278.2017.
- Gesheng Cheng; Lu He; Yushun Zhang; LincRNA-Cox2 promotes pulmonary arterial hypertension by regulating the let-7a-mediated STAT3 signaling pathway. Molecular and Cellular Biochemistry 2020, 475, 239-247, 10.1007/s11010-020-03877-6.
- Jiacai Hu; Zhijie Yang; Hao Wu; Daochun Wang; Rhein attenuates renal inflammatory injury of uric acid nephropathy via lincRNA-Cox2/miR-150-5p/STAT1 axis. International Immunopharmacology 2020, 85, 106620, 10.1016/j.intimp.2020.106620.
- Jun Zhao; Jinding Pu; Binwei Hao; Lingmei Huang; Jinglong Chen; Wei Hong; Yumin Zhou; Bing Li; Pixin Ran; LncRNA RP11-86H7.1 promotes airway inflammation induced by TRAPM2.5 by acting as a ceRNA of miRNA-9-5p to regulate NFKB1 in HBECS. Scientific Reports 2020, 10, 1, 10.1038/s41598-020-68327-1.
- Qin Shen; Jiao Zheng; Xueling Wang; Wen Hu; Yongjie Jiang; Yongliang Jiang; LncRNA SNHG5 regulates cell apoptosis and inflammation by miR-132/PTEN axis in COPD. Biomedicine & Pharmacotherapy 2020, 126, 110016, 10.1016/j.biopha.2020.110016.
- Keith E. Shearwin; Benjamin P. Callen; J. Barry Egan; Transcriptional interference – a crash course. Trends in Genetics 2005, 21, 339-345, 10.1016/j.tig.2005.04.009.
- Miki Ebisuya; Takuya Yamamoto; May Nakajima; Eisuke Nishida; Ripples from neighbouring transcription. Nature 2008, 10, 1106-1113, 10.1038/ncb1771.
- Jesse M. Engreitz; Jenna E. Haines; Elizabeth Perez; Glen Munson; Jenny Chen; Michael Kane; Patrick E. McDonel; Mitchell Guttman; Eric S. Lander; Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016, 539, 452-455, 10.1038/nature20149.
- Guoliang Li; Xiaoan Ruan; Raymond K. Auerbach; Kuljeet Singh Sandhu; Meizhen Zheng; Ping Wang; Huay Mei Poh; Yufen Goh; Joanne Lim; Jingyao Zhang; et al. Extensive Promoter-Centered Chromatin Interactions Provide a Topological Basis for Transcription Regulation. Cell 2012, 148, 84-98, 10.1016/j.cell.2011.12.014.
- Vikram R. Paralkar; Cristian C. Taborda; Peng Huang; Yu Yao; Andrew V. Kossenkov; Rishi Prasad; Jing Luan; James Davies; Jim R. Hughes; Ross Hardison; et al. Unlinking an lncRNA from Its Associated cis Element. Molecular Cell 2016, 62, 104-110, 10.1016/j.molcel.2016.02.029.
- David Artis; Hergen Spits; The biology of innate lymphoid cells. Nature 2015, 517, 293-301, 10.1038/nature14189.
- Yiliang Wang; Lianzhou Huang; Yuan Wang; Weisheng Luo; Feng Li; Ji Xiao; Shurong Qin; Zhaoyang Wang; Xiaowei Song; Fujun Jin; et al. Single-cell RNA-sequencing analysis identifies host long noncoding RNA MAMDC2-AS1 as a co-factor for HSV-1 nuclear transport. International Journal of Biological Sciences 2020, 16, 1586-1603, 10.7150/ijbs.42556.
- Lei Sun; Loyal A. Goff; Cole Trapnell; Ryan Alexander; Kinyui Alice Lo; Ezgi Hacisuleyman; Martin Sauvageau; Barbara Tazon-Vega; David R. Kelley; David G. Hendrickson; et al. Long noncoding RNAs regulate adipogenesis. Proceedings of the National Academy of Sciences 2013, 110, 3387-3392, 10.1073/pnas.1222643110.
- Markus Kretz; Zurab Siprashvili; Ci Chu; Dan Webster; Ashley Zehnder; Kun Qu; Carolyn S. Lee; Ross J. Flockhart; Abigail F. Groff; Jennifer Chow; et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature 2012, 493, 231-235, 10.1038/nature11661.
- Carmen Lanzillotti; Monica De Mattei; Chiara Mazziotta; Francesca Taraballi; John Charles Rotondo; Mauro Tognon; Fernanda Martini; Long Non-coding RNAs and MicroRNAs Interplay in Osteogenic Differentiation of Mesenchymal Stem Cells. Frontiers in Cell and Developmental Biology 2021, 9, 742, 10.3389/fcell.2021.646032.
- Motonari Kondo; Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunological Reviews 2010, 238, 37-46, 10.1111/j.1600-065x.2010.00963.x.
- Bruce Beutler; Innate immunity: an overview. Molecular Immunology 2004, 40, 845-859, 10.1016/j.molimm.2003.10.005.
- Massimo Locati; Alberto Mantovani; Antonio Sica; Macrophage Activation and Polarization as an Adaptive Component of Innate Immunity. Advances in Immunology 2013, 120, 163-184, 10.1016/b978-0-12-417028-5.00006-5.
- Ming-Tai Chen; Hai-Shuang Lin; Chao Shen; Yan-Ni Ma; Fang Wang; Hua-Lu Zhao; Jia Yu; Jun-Wu Zhang; The PU.1-Regulated Long Noncoding RNA Lnc-MC Controls Human Monocyte/Macrophage Differentiation through Interaction with MicroRNA-199a-5p. Molecular and Cellular Biology 2015, 35, MCB.00429-15, 10.1128/mcb.00429-15.
- Chin-An Yang; Ju-Pi Li; Ju-Chen Yen; I-Lu Lai; Yu-Chen Ho; Yu-Chia Chen; Joung-Liang Lan; Jan-Gowth Chang; lncRNA NTT/PBOV1 Axis Promotes Monocyte Differentiation and Is Elevated in Rheumatoid Arthritis. International Journal of Molecular Sciences 2018, 19, 2806, 10.3390/ijms19092806.
- Yibiao Ye; Yunxiuxiu Xu; Yu Lai; Wenguang He; Yanshan Li; Ruomei Wang; Xinxi Luo; Rufu Chen; Tao Chen; Long non‐coding RNA cox‐2 prevents immune evasion and metastasis of hepatocellular carcinoma by altering M1/M2 macrophage polarization. Journal of Cellular Biochemistry 2017, 119, 2951-2963, 10.1002/jcb.26509.
- Xiaowen Chi; Beichen Ding; Lijuan Zhang; Jiawen Zhang; Jianmei Wang; Wei Zhang; lncRNA GAS5 promotes M1 macrophage polarization via miR‐455‐5p/SOCS3 pathway in childhood pneumonia. Journal of Cellular Physiology 2018, 234, 13242-13251, 10.1002/jcp.27996.
- Nannan Li; Yuan Liu; Jingfen Cai; LncRNA MIR155HG regulates M1/M2 macrophage polarization in chronic obstructive pulmonary disease. Biomedicine & Pharmacotherapy 2019, 117, 109015, 10.1016/j.biopha.2019.109015.
- Meng Du; Lin Yuan; Xin Tan; Dandan Huang; Xiaojing Wang; Zhe Zheng; Xiaoxiang Mao; Xiangrao Li; Liu Yang; Kun Huang; et al. The LPS-inducible lncRNA Mirt2 is a negative regulator of inflammation. Nature Communications 2017, 8, 1-18, 10.1038/s41467-017-02229-1.
- Cao, J.; Dong, R.; Jiang, L.; Gong, Y.; Yuan, M.; You, J.; Meng,W.; Chen, Z.; Zhang, N.; Weng, Q; et al. LncRNA-MM2P identified as a modulator of macrophage M2 polarization. Cancer Immunol. Res 2019, 7, 292–305, 10.1158/2326-6066.
- Xiao Han; Saihua Huang; Ping Xue; Jinrong Fu; Lijuan Liu; Caiyan Zhang; Lan Yang; Li Xia; Licheng Sun; Shau-Ku Huang; et al. LncRNAPTPRE-AS1modulates M2 macrophage activation and inflammatory diseases by epigenetic promotion of PTPRE. Science Advances 2019, 5, eaax9230, 10.1126/sciadv.aax9230.
- Pin Wang; Yiquan Xue; Yanmei Han; L. Lin; Cong Wu; Sheng Xu; ZhengPing Jiang; Junfang Xu; Qiuyan Liu; Xuetao Cao; et al. The STAT3-Binding Long Noncoding RNA lnc-DC Controls Human Dendritic Cell Differentiation. Science 2014, 344, 310-313, 10.1126/science.1251456.
- Jian Wu; Hanlu Zhang; Yang Zheng; Xiangyuan Jin; Mingyang Liu; Shuang Li; Qi Zhao; Xianglan Liu; Yongshun Wang; Ming Shi; et al. The Long Noncoding RNA MALAT1 Induces Tolerogenic Dendritic Cells and Regulatory T Cells via miR155/Dendritic Cell-Specific Intercellular Adhesion Molecule-3 Grabbing Nonintegrin/IL10 Axis. Frontiers in Immunology 2018, 9, 1847, 10.3389/fimmu.2018.01847.
- Xueqing Zhang; Zheng Lian; Carolyn Padden; Mark B. Gerstein; Joel Rozowsky; Michael Snyder; Thomas R. Gingeras; Philipp Kapranov; Sherman M. Weissman; Peter E. Newburger; et al. A myelopoiesis-associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood 2009, 113, 2526-2534, 10.1182/blood-2008-06-162164.
- Ruya Zhang; Fang Ni; Binqing Fu; Yang Wu; Rui Sun; Zhigang Tian; Haiming Wei; A long noncoding RNA positively regulates CD56 in human natural killer cells. Oncotarget 2016, 7, 72546-72558, 10.18632/oncotarget.12466.
- Peipei Fang; Luxia Xiang; Weilai Chen; Shaoxun Li; Shanshan Huang; Jie Li; Lu Zhuge; Lingxiang Jin; Wenke Feng; Yiping Chen; et al. LncRNA GAS5 enhanced the killing effect of NK cell on liver cancer through regulating miR-544/RUNX3. Innate Immunity 2019, 25, 99-109, 10.1177/1753425919827632.
- Shuang Li; Anjing Zhu; Kai Ren; Shilin Li; Limin Chen; IFNβ-induced exosomal linc-EPHA6-1 promotes cytotoxicity of NK cells by acting as a ceRNA for hsa-miR-4485-5p to up-regulate NKp46 expression. Life Sciences 2020, 257, 118064, 10.1016/j.lfs.2020.118064.
- Subhra K Biswas; Alberto Mantovani; Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature Immunology 2010, 11, 889-896, 10.1038/ni.1937.
- Breno C.B. Beirão; Teresa Raposo; Lisa Y. Pang; David J. Argyle; Canine mammary cancer cells direct macrophages toward an intermediate activation state between M1/M2. BMC Veterinary Research 2015, 11, 1-14, 10.1186/s12917-015-0473-y.
- Xiaohua Mao; Zhenyi Su; Adnan K. Mookhtiar; Long non-coding RNA: a versatile regulator of the nuclear factor-κB signalling circuit. Immunology 2017, 150, 379-388, 10.1111/imm.12698.
- Ai-Ping Mao; Jun Shen; Zhixiang Zuo; Expression and regulation of long noncoding RNAs in TLR4 signaling in mouse macrophages. BMC Genomics 2015, 16, 1-14, 10.1186/s12864-015-1270-5.
- Alberto Mantovani; Antonio Sica; Silvano Sozzani; Paola Allavena; Annunciata Vecchi; Massimo Locati; The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology 2004, 25, 677-686, 10.1016/j.it.2004.09.015.
- Steinman, R.M.; Hemmi, H. . Dendritic Cells: Translating Innate to Adaptive Immunity; Springer: Berlin/Heidelberg, Germany,, 2006; pp. pp. 17–58.
- Susanne Viktoria Schmidt; Andrea Cecilia Nino-Castro; Joachim L. Schultze; Regulatory dendritic cells: there is more than just immune activation. Frontiers in Immunology 2012, 3, 274, 10.3389/fimmu.2012.00274.
- Akam, M.; Hox genes and the evolution of diverse body plans. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1995, 349, 313–319, 10.1098/rstb.1995.0119.
- Ling Bei; Yufeng Lu; Susan L. Bellis; Wei Zhou; Elizabeth Horvath; Elizabeth A. Eklund; Identification of a HoxA10 Activation Domain Necessary for Transcription of the Gene Encoding β3 Integrin during Myeloid Differentiation. Journal of Biological Chemistry 2007, 282, 16846-16859, 10.1074/jbc.m609744200.
- Kim L. Rice; Jonathan D. Licht; HOX deregulation in acute myeloid leukemia. Journal of Clinical Investigation 2007, 117, 865-868, 10.1172/jci31861.
- Michael A. Caligiuri; Human natural killer cells. Blood 2008, 112, 461-469, 10.1182/blood-2007-09-077438.
- Heleen H. Van Acker; Anna Capsomidis; Evelien Smits; Viggo F. Van Tendeloo; CD56 in the Immune System: More Than a Marker for Cytotoxicity?. Frontiers in Immunology 2017, 8, 892, 10.3389/fimmu.2017.00892.
- Marco E. Bianchi; DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of Leukocyte Biology 2006, 81, 1-5, 10.1189/jlb.0306164.
- Mona Motwani; Scott Pesiridis; Katherine A. Fitzgerald; DNA sensing by the cGAS–STING pathway in health and disease. Nature Reviews Genetics 2019, 20, 657-674, 10.1038/s41576-019-0151-1.
- Sky W. Brubaker; Kevin S. Bonham; Ivan Zanoni; Jonathan C. Kagan; Innate Immune Pattern Recognition: A Cell Biological Perspective. Annual Review of Immunology 2015, 33, 257-290, 10.1146/annurev-immunol-032414-112240.
- Yihui Fan; Renfang Mao; Jianhua Yang; NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein & Cell 2013, 4, 176-185, 10.1007/s13238-013-2084-3.
- Frank X. Zhang; Carsten J. Kirschning; Roberta Mancinelli; Xiao-Ping Xu; Yiping Jin; Emmanuelle Faure; Alberto Mantovani; Mike Rothe; Marta Muzio; Moshe Arditi; et al. Bacterial Lipopolysaccharide Activates Nuclear Factor-κB through Interleukin-1 Signaling Mediators in Cultured Human Dermal Endothelial Cells and Mononuclear Phagocytes. Journal of Biological Chemistry 1999, 274, 7611-7614, 10.1074/jbc.274.12.7611.
- Carl Nathan; Points of control in inflammation. Nature 2002, 420, 846-852, 10.1038/nature01320.
- Zilong Wen; Zhong Zhong; James E Darnell; Maximal activation of transcription by statl and stat3 requires both tyrosine and serine phosphorylation. Cell 1995, 82, 241-250, 10.1016/0092-8674(95)90311-9.
- Guobin He; Guann-Yi Yu; Vladislav Temkin; Hisanobu Ogata; Christian Kuntzen; Toshiharu Sakurai; Wolfgang Sieghart; Markus Peck-Radosavljevic; Hyam L. Leffert; Michael Karin; et al. Hepatocyte IKKβ/NF-κB Inhibits Tumor Promotion and Progression by Preventing Oxidative Stress-Driven STAT3 Activation. Cancer Cell 2010, 17, 286-297, 10.1016/j.ccr.2009.12.048.
- Rehan Ahmad; Hasan Rajabi; Michio Kosugi; Maya Datt Joshi; Maroof Alam; Baldev Vasir; Takeshi Kawano; Surender Kharbanda; Donald Kufe; MUC1-C Oncoprotein Promotes STAT3 Activation in an Autoinductive Regulatory Loop. Science Signaling 2011, 4, ra9-ra9, 10.1126/scisignal.2001426.
- Marcelo Freire; Thomas Van Dyke; Natural resolution of inflammation. Periodontology 2000 2013, 63, 149-164, 10.1111/prd.12034.
- Mitchell Guttman; Ido Amit; Manuel Garber; Courtney French; Michael F. Lin; David Feldser; Maite Huarte; Or Zuk; Bryce W. Carey; John P. Cassady; et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 2009, 458, 223-227, 10.1038/nature07672.
- Guoku Hu; Ai-Yu Gong; Yang Wang; Shibin Ma; Xiqiang Chen; Jing Chen; Chun-Jen Su; Annemarie Shibata; Juliane K. Strauss-Soukup; Kristen M. Drescher; et al. LincRNA-Cox2 Promotes Late Inflammatory Gene Transcription in Macrophages through Modulating SWI/SNF-Mediated Chromatin Remodeling. The Journal of Immunology 2016, 196, 2799-2808, 10.4049/jimmunol.1502146.
- Mohamed Maarouf; Biao Chen; Yuhai Chen; Xuefei Wang; Kul Raj Rai; Zhonghui Zhao; Shasha Liu; Yingying Li; Meng Xiao; Ji‐Long Chen; et al. Identification of lncRNA‐155 encoded by MIR155HG as a novel regulator of innate immunity against influenza A virus infection. Cellular Microbiology 2019, 21, e13036, 10.1111/cmi.13036.
- Zhonghan Li; Ti-Chun Chao; Kung-Yen Chang; Nianwei Lin; Veena S. Patil; Chisato Shimizu; Steven R. Head; Jane C. Burns; Tariq M. Rana; The long noncoding RNA THRIL regulates TNF expression through its interaction with hnRNPL. Proceedings of the National Academy of Sciences 2013, 111, 1002-1007, 10.1073/pnas.1313768111.
- Wei‐Jun Liang; Xiao‐Yuan Zeng; Sha‐Li Jiang; Hong‐Yi Tan; Mu‐Yun Yan; Hong‐Zhong Yang; Long non‐coding RNA MALAT1 sponges miR‐149 to promote inflammatory responses of LPS‐induced acute lung injury by targeting MyD88. Cell Biology International 2019, 44, 317-326, 10.1002/cbin.11235.
- Henan Xu; Yan Jiang; Xiaoqing Xu; Xiaoping Su; Yang Liu; Yuanwu Ma; Yong Zhao; Zhongyang Shen; Bo Huang; Xuetao Cao; et al. Inducible degradation of lncRNA Sros1 promotes IFN-γ-mediated activation of innate immune responses by stabilizing Stat1 mRNA. Nature Immunology 2019, 20, 1621-1630, 10.1038/s41590-019-0542-7.
- Yuhai Chen; Jiayue Hu; Shasha Liu; Biao Chen; Meng Xiao; Yingying Li; Yuan Liao; Kul Raj Rai; Zhonghui Zhao; Jing Ouyang; et al. RDUR, a lncRNA, Promotes Innate Antiviral Responses and Provides Feedback Control of NF-κB Activation. Frontiers in Immunology 2021, 12, 1849, 10.3389/fimmu.2021.672165.
- Pengfei Zhang; Limian Cao; Rongbin Zhou; Xiaolu Yang; Mian Wu; The lncRNA Neat1 promotes activation of inflammasomes in macrophages. Nature Communications 2019, 10, 1-17, 10.1038/s41467-019-09482-6.
- Katsutoshi Imamura; Naoto Imamachi; Gen Akizuki; Michiko Kumakura; Yasushi Kawaguchi; Kyosuke Nagata; Akihisa Kato; Hiroki Sato; Misako Yoneda; Chieko Kai; et al. Long Noncoding RNA NEAT1-Dependent SFPQ Relocation from Promoter Region to Paraspeckle Mediates IL8 Expression upon Immune Stimuli. Molecular Cell 2014, 53, 393-406, 10.1016/j.molcel.2014.01.009.
- Hongyu Lin; Minghong Jiang; Lun Liu; Zongheng Yang; Zhongfei Ma; Shuo Liu; Yuanwu Ma; Lianfeng Zhang; Xuetao Cao; The long noncoding RNA Lnczc3h7a promotes a TRIM25-mediated RIG-I antiviral innate immune response. Nature Immunology 2019, 20, 812-823, 10.1038/s41590-019-0379-0.
- Yi Wang; Zhiting Huo; Quanshi Lin; Yuxia Lin; Cancan Chen; Yanxia Huang; Changbai Huang; Junsong Zhang; Junfang He; Chao Liu; et al. Positive Feedback Loop of Long Noncoding RNA OASL-IT1 and Innate Immune Response Restricts the Replication of Zika Virus in Epithelial A549 Cells. Journal of Innate Immunity 2021, 13, 179-193, 10.1159/000513606.
- Ainara Castellanos-Rubio; Nora Fernandez-Jimenez; Radomir Kratchmarov; Xiaobing Luo; Govind Bhagat; Peter H. R. Green; Robert Schneider; Megerditch Kiledjian; Jose Ramon Bilbao; Sankar Ghosh; et al. A long noncoding RNA associated with susceptibility to celiac disease. Science 2016, 352, 91-95, 10.1126/science.aad0467.
- Nicole A Rapicavoli; Kun Qu; Jiajing Zhang; Megan Mikhail; Remi-Martin Laberge; Howard Y Chang; A mammalian pseudogene lncRNA at the interface of inflammation and anti-inflammatory therapeutics. eLife 2013, 2, e00762, 10.7554/elife.00762.
- Gui Zhao; Zhenyi Su; Dan Song; Yimin Mao; Xiaohua Mao; The long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced inflammatory response through its interaction with NF-κB. FEBS Letters 2016, 590, 2884-2895, 10.1002/1873-3468.12315.
- Jingjing Fan; Min Cheng; Xiaojing Chi; Xiuying Liu; Wei Yang; A Human Long Non-coding RNA LncATV Promotes Virus Replication Through Restricting RIG-I–Mediated Innate Immunity. Frontiers in Immunology 2019, 10, 1711, 10.3389/fimmu.2019.01711.
- Minghong Jiang; Shikun Zhang; Zongheng Yang; Hongyu Lin; Jun Zhu; Lun Liu; Wendie Wang; Shuo Liu; Wei Liu; Yuanwu Ma; et al. Self-Recognition of an Inducible Host lncRNA by RIG-I Feedback Restricts Innate Immune Response. Cell 2018, 173, 906-919.e13, 10.1016/j.cell.2018.03.064.
- Jing Ouyang; Xiaomei Zhu; Yuhai Chen; Haitao Wei; Qinghuang Chen; Xiaojuan Chi; Baomin Qi; Lianfeng Zhang; Yi Zhao; George Fu Gao; et al. NRAV, a Long Noncoding RNA, Modulates Antiviral Responses through Suppression of Interferon-Stimulated Gene Transcription. Cell Host & Microbe 2014, 16, 616-626, 10.1016/j.chom.2014.10.001.
- Yunhui Jia; Yuanyuan Wei; Modulators of MicroRNA Function in the Immune System. International Journal of Molecular Sciences 2020, 21, 2357, 10.3390/ijms21072357.
- Carlos Zgheib; Maggie M. Hodges; Junyi Hu; Kenneth W. Liechty; Junwang Xu; Long non-coding RNA Lethe regulates hyperglycemia-induced reactive oxygen species production in macrophages. PLoS ONE 2017, 12, e0177453, 10.1371/journal.pone.0177453.
- Jan Rehwinkel; Caetano Reis e Sousa; RIGorous Detection: Exposing Virus Through RNA Sensing. Science 2010, 327, 284-286, 10.1126/science.1185068.
- Lena Alexopoulou; Agnieszka Czopik Holt; Ruslan Medzhitov; Richard A. Flavell; Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 2001, 413, 732-738, 10.1038/35099560.
- Florian Heil; Hiroaki Hemmi; Hubertus Hochrein; Franziska Ampenberger; Carsten Kirschning; Shizuo Akira; Grayson Lipford; Hermann Wagner; Stefan Bauer; Species-Specific Recognition of Single-Stranded RNA via Toll-like Receptor 7 and 8. Science 2004, 303, 1526-1529, 10.1126/science.1093620.
- Umeharu Ohto; Hanako Ishida; Takuma Shibata; Ryota Sato; Kensuke Miyake; Toshiyuki Shimizu; Toll-like Receptor 9 Contains Two DNA Binding Sites that Function Cooperatively to Promote Receptor Dimerization and Activation. Immunity 2018, 48, 649-658.e4, 10.1016/j.immuni.2018.03.013.
- Kwan T Chow; Michael Gale; Yueh-Ming Loo; RIG-I and Other RNA Sensors in Antiviral Immunity. Annual Review of Immunology 2018, 36, 667-694, 10.1146/annurev-immunol-042617-053309.
- Andrea Ablasser; Marion Goldeck; Taner Cavlar; Tobias Deimling; Gregor Witte; Ingo Röhl; Karl-Peter Hopfner; Janos Ludwig; Veit Hornung; cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013, 498, 380-384, 10.1038/nature12306.
- Alvin Lu; Venkat Giri Magupalli; Jianbin Ruan; Qian Yin; Maninjay K. Atianand; Matthijn R. Vos; Gunnar Schröder; Kate Fitzgerald; Hao Wu; Edward Egelman; et al. Unified Polymerization Mechanism for the Assembly of ASC-Dependent Inflammasomes. Cell 2014, 156, 1193-1206, 10.1016/j.cell.2014.02.008.
- Petr Broz; Vishva M. Dixit; Inflammasomes: mechanism of assembly, regulation and signalling. Nature Reviews Immunology 2016, 16, 407-420, 10.1038/nri.2016.58.
- Wei Liu; Ziqiao Wang; Lun Liu; Zongheng Yang; Shuo Liu; Zhongfei Ma; Yin Liu; Yuanwu Ma; Lianfeng Zhang; Xuan Zhang; et al. LncRNAMalat1inhibition of TDP43 cleavage suppresses IRF3-initiated antiviral innate immunity. Proceedings of the National Academy of Sciences 2020, 117, 23695-23706, 10.1073/pnas.2003932117.
- Luigi Ferrucci; Elisa Fabbri; Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology 2018, 15, 505-522, 10.1038/s41569-018-0064-2.
- Vinod Kumar; Harm-Jan Westra; Juha Karjalainen; Daria V. Zhernakova; Tonu Esko; Barbara Hrdlickova; Rodrigo Almeida; Alexandra Zhernakova; Eva Reinmaa; Urmo Võsa; et al. Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA Expression. PLOS Genetics 2013, 9, e1003201, 10.1371/journal.pgen.1003201.
- Isis Ricaño-Ponce; Cisca Wijmenga; Mapping of Immune-Mediated Disease Genes. Annual Review of Genomics and Human Genetics 2013, 14, 325-353, 10.1146/annurev-genom-091212-153450.
- Markus Manz; Steffen Boettcher; Emergency granulopoiesis. Nature Reviews Immunology 2014, 14, 302-314, 10.1038/nri3660.
- Florent Ginhoux; Steffen Jung; Monocytes and macrophages: developmental pathways and tissue homeostasis. Nature Reviews Immunology 2014, 14, 392-404, 10.1038/nri3671.
- Jonathan J. Kotzin; Sean Spencer; Sam McCright; Dinesh B. Uthaya Kumar; Magalie A. Collet; Walter Mowel; Ellen N. Elliott; Asli Uyar; Michelle Makiya; Margaret C. Dunagin; et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 2016, 537, 239-243, 10.1038/nature19346.
- Chengwu Zeng; Yan Xu; Ling Xu; Xibao Yu; Jingjing Cheng; Lijian Yang; Shaohua Chen; Yangqiu Li; Inhibition of long non-coding RNA NEAT1 impairs myeloid differentiation in acute promyelocytic leukemia cells. BMC Cancer 2014, 14, 1-7, 10.1186/1471-2407-14-693.
- Junyu Liang; Weiqian Chen; Jin Lin; LncRNA: An all-rounder in rheumatoid arthritis. Journal of Translational Internal Medicine 2019, 7, 3-9, 10.2478/jtim-2019-0002.
- Jinsoo Song; DongKyun Kim; Jiyeon Han; Yunha Kim; Myeungsu Lee; Eun-Jung Jin; PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clinical and Experimental Medicine 2014, 15, 121-126, 10.1007/s10238-013-0271-4.
- Cheng Liu; Xiaojun Guo; Sunpeng Bai; Guangjun Zeng; Hao Wang; lncRNA CASC2 downregulation participates in rheumatoid arthritis, and CASC2 overexpression promotes the apoptosis of fibroblast‑like synoviocytes by downregulating IL‑17. Molecular Medicine Reports 2020, 21, 2131-2137, 10.3892/mmr.2020.11018.
- Guo-Ping Shi; Ilze Bot; Petri T. Kovanen; Mast cells in human and experimental cardiometabolic diseases. Nature Reviews Cardiology 2015, 12, 643-658, 10.1038/nrcardio.2015.117.
- Andrew J Kim; Na Xu; Katherine E Yutzey; Macrophage lineages in heart valve development and disease. Cardiovascular Research 2020, 117, 663-673, 10.1093/cvr/cvaa062.
- Changbin Sun; Yahong Fu; Xia Gu; Xiangwen Xi; Xiang Peng; Chuhan Wang; Qi Sun; Xueyu Wang; Fengcui Qian; Zhifeng Qin; et al. Macrophage-Enriched lncRNA RAPIA. Arteriosclerosis, Thrombosis, and Vascular Biology 2020, 40, 1464-1478, 10.1161/atvbaha.119.313749.
- Viorel Simion; Haoyang Zhou; Stefan Haemmig; Jacob B. Pierce; Shanelle Mendes; Yevgenia Tesmenitsky; Daniel Pérez-Cremades; James F. Lee; Alex F. Chen; Nicoletta Ronda; et al. A macrophage-specific lncRNA regulates apoptosis and atherosclerosis by tethering HuR in the nucleus. Nature Communications 2020, 11, 1-16, 10.1038/s41467-020-19664-2.
- Zi-Ming Ye; Shuai Yang; Yuan-Peng Xia; Rui-Ting Hu; Shengcai Chen; Bo-Wei Li; Shao-Li Chen; Xue-Ying Luo; Ling Mao; Yanan Li; et al. LncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death & Disease 2019, 10, 1-16, 10.1038/s41419-019-1409-4.
- John Hung; Jessica P. Scanlon; Amira D. Mahmoud; Julie Rodor; Margaret Ballantyne; Margaux A.C. Fontaine; Lieve Temmerman; Jakub Kaczynski; Katie L. Connor; Raghu Bhushan; et al. Novel Plaque Enriched Long Noncoding RNA in Atherosclerotic Macrophage Regulation (PELATON). Arteriosclerosis, Thrombosis, and Vascular Biology 2020, 40, 697-713, 10.1161/atvbaha.119.313430.
- Shuguo Yang; Jingang Sun; LncRNA SRA deregulation contributes to the development of atherosclerosis by causing dysfunction of endothelial cells through repressing the expression of adipose triglyceride lipase. Molecular Medicine Reports 2018, 18, 5207-5214, 10.3892/mmr.2018.9497.
- Li-Mei Wu; Shao-Guo Wu; Fei Chen; Qian Wu; Chang-Meng Wu; Chun-Min Kang; Xin He; Ru-Yi Zhang; Zhi-Feng Lu; Xue-Heng Li; et al. Atorvastatin inhibits pyroptosis through the lncRNA NEXN-AS1/NEXN pathway in human vascular endothelial cells. Atherosclerosis 2020, 293, 26-34, 10.1016/j.atherosclerosis.2019.11.033.
- Nadiya Khyzha; Melvin Khor; Peter V. DiStefano; Liangxi Wang; Ljubica Matic; Ulf Hedin; Michael D. Wilson; Lars Maegdefessel; Jason E. Fish; Regulation of CCL2 expression in human vascular endothelial cells by a neighboring divergently transcribed long noncoding RNA. Proceedings of the National Academy of Sciences 2019, 116, 16410-16419, 10.1073/pnas.1904108116.
- Qiong Zhang; Ti‐Chun Chao; Veena S Patil; Yue Qin; Shashi Kant Tiwari; Joshua Chiou; Alexander Dobin; Chih‐Ming Tsai; Zhonghan Li; Jason Dang; et al. The long noncoding RNA ROCKI regulates inflammatory gene expression. The EMBO Journal 2019, 38, e100041, 10.15252/embj.2018100041.
- Yongsheng Quan; Kerui Song; Yan Zhang; Changxin Zhu; Zhaohua Shen; Shuai Wu; Weiwei Luo; Bei Tan; Zhenyu Yang; Xiaoyan Wang; et al. Roseburia intestinalis -derived flagellin is a negative regulator of intestinal inflammation. Biochemical and Biophysical Research Communications 2018, 501, 791-799, 10.1016/j.bbrc.2018.05.075.
- Cuixia Qiao; Lili Yang; Jine Wan; Xiaoling Liu; Chengjian Pang; Wenli You; Gang Zhao; Long noncoding RNA ANRIL contributes to the development of ulcerative colitis by miR-323b-5p/TLR4/MyD88/NF-κB pathway. Biochemical and Biophysical Research Communications 2018, 508, 217-224, 10.1016/j.bbrc.2018.11.100.
- Gosia Trynka; Spanish Consortium on the Genetics of Coeliac Disease (CEGEC); Karen A Hunt; Nicholas Bockett; Jihane Romanos; Vanisha Mistry; Agata Szperl; Sjoerd F Bakker; Maria Teresa Bardella; Leena Bhaw-Rosun; et al. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nature Genetics 2011, 43, 1193-1201, 10.1038/ng.998.
- N. Fernandez-Jimenez; A. Castellanos-Rubio; L. Plaza-Izurieta; I. Irastorza; X. Elcoroaristizabal; Amaia Jauregi Miguel; T. Lopez-Euba; C. Tutau; Maria De Los Angeles Martinez De Pancorbo; Jc Vitoria; et al. Coregulation and modulation of NF B-related genes in celiac disease: uncovered aspects of gut mucosal inflammation. Human Molecular Genetics 2013, 23, 1298-1310, 10.1093/hmg/ddt520.
- Elisa Gnodi; Clara Mancuso; Luca Elli; Elisa Ballarini; Raffaella Meneveri; Jean Beaulieu; Donatella Barisani; Gliadin, through the Activation of Innate Immunity, Triggers lncRNA NEAT1 Expression in Celiac Disease Duodenal Mucosa. International Journal of Molecular Sciences 2021, 22, 1289, 10.3390/ijms22031289.
- J-Y Liu; J Yao; X-M Li; Y-C Song; X-Q Wang; Y-J Li; Bin Yan; Q Jiang; Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death & Disease 2014, 5, e1506-e1506, 10.1038/cddis.2014.466.
- Hong Wu; Fang Wen; Mei Jiang; Qiang Liu; Yijun Nie; LncRNA uc.48+ is involved in the diabetic immune and inflammatory responses mediated by P2X7 receptor in RAW264.7 macrophages. International Journal of Molecular Medicine 2018, 42, 1152-1160, 10.3892/ijmm.2018.3661.
- Marpadga A. Reddy; Zhuo Chen; Jung Tak Park; Mei Wang; Linda Lanting; Qiang Zhang; Kirti Bhatt; Amy Leung; Xiwei Wu; Sumanth Putta; et al. Regulation of Inflammatory Phenotype in Macrophages by a Diabetes-Induced Long Noncoding RNA. Diabetes 2014, 63, 4249-4261, 10.2337/db14-0298.
- Chandrakumar Sathishkumar; Paramasivam Prabu; Viswanathan Mohan; Muthuswamy Balasubramanyam; Linking a role of lncRNAs (long non-coding RNAs) with insulin resistance, accelerated senescence, and inflammation in patients with type 2 diabetes. Human Genomics 2018, 12, 1-9, 10.1186/s40246-018-0173-3.
